
v 6.1 (Operative 11/9/22)
Sampling and
Testing
Metrc Guide
Table of Contents
General Rules and Guidelines ................................................................................................................................................................1
Definitions of Lots and Batches .............................................................................................................................................................2
Usable Marijuana Batch Testing ........................................................................................................................................... 2
Extract, Concentrate, and Cannabinoid Product Batch Testing ............................................................................................ 6
Identifying Lots and Batches ..................................................................................................................................................................8
Creating Samples ...................................................................................................................................................................................9
Entering Test Results ........................................................................................................................................................................... 12
Entering results for extract, concentrate, and cannabinoid product increments ................................................................ 15
Retesting and Remediation ................................................................................................................................................................. 17
Retesting ............................................................................................................................................................................. 17
Remediation ........................................................................................................................................................................ 20
Research and Development Testing ..................................................................................................................................................... 22
Tentatively Identified Compounds (TICs) ............................................................................................................................................. 23
Reconciling Weight Taken for Testing (Adjustments) ........................................................................................................................... 24
Lab Subcontracting .............................................................................................................................................................................. 26
Testing Inhalable Hemp Vapor Items from non-OLCC licensees ........................................................................................................... 29
Appendix 1: Frequently Asked Questions ............................................................................................................................................ 32
I want to combine multiple packages into one batch for purposes of testing – can I submit them for testing together?
........................................................................................................................................................................................ 32
Is a lab able to conduct “extra” tests like terpenes not covered by the testing rules? How should the sampling and
test results be recorded in Metrc? ................................................................................................................................. 32
Can a lab test for potency as part of quality control and not have it “count” for purposes of state testing? What about
for pesticides? ................................................................................................................................................................. 32
Test results were entered, but the status still says “TestingInProgress” – why? ........................................................... 33
A lab tested my sample and gave me a PDF report of my test results, but my package in Metrc still says
“NotSubmitted” – can I still transfer my product? ......................................................................................................... 33
My product failed a test. What are my options? ............................................................................................................ 33
My harvest lot has more than one batch, and each batch has a slightly different THC content – how should I label my
product? .......................................................................................................................................................................... 34
If a lab goes out to sample at the site of a producer or processor how should the data be recorded in Metrc? .......... 34
If a producer brings in a 50.0 pound batch for sampling how should this be recorded in Metrc? ................................. 34
Can samples be stored by a lab at a remote, unlicensed facility? .................................................................................. 34
If I am a licensed lab can I have my employees collect samples? ................................................................................... 35
Are there options other than manual data entry for inputting information into Metrc? .............................................. 35
How many scales do I need? Do they need to be certified? ........................................................................................... 35
Where can I go or who should I ask if I have more questions? ...................................................................................... 35
Appendix 2: Testing Requirements ................................................................................................................................................ 37
Appendix 3: Cannabis Failed Testing Guide.......................................................................................................................................... 41
Appendix 4: How to Enter Test Results ................................................................................................................................................ 43

1
General Rules and Guidelines
Rules and requirements related to handling and testing of cannabis is overseen by three state agencies.
The Oregon Liquor Control Commission (OLCC) approves licenses for the recreational marijuana
market and oversees recreational marijuana rules, including security requirements; data
tracking in Metrc, the state’s Cannabis Tracking System; and proper handling of marijuana items
produced and sold within the recreational market.
The Oregon Health Authority (OHA) oversees the Oregon Medical Marijuana Program,
establishes rules for all testing of marijuana items (for both medical and recreational sales), and
regulates the production and sales of medical marijuana items in the OHA system. Within OHA
is also the Oregon Environmental Laboratory Accreditation Program (ORELAP), which oversees
sampling and testing of all cannabis – medical marijuana, recreational marijuana, and hemp.
ORELAP accreditation is required for OLCC-licensure, and only an OLCC-licensed and OHA-
accredited laboratory can conduct state-required tests.
The Oregon Department of Agriculture (ODA) oversees the Oregon hemp program; monitors
and regulates pesticide use on cannabis products; and, through the Weights and Measures
Program, certifies non-sampling scales for recreational marijuana licensees for all weights
entered into Metrc.
This guide is specific to rules and data tracking within the recreational marijuana market. All recreational
marijuana items available for sale to consumers must be tested as laid out in OHA’s testing rules.
The
types of tests required depends on the product being tested and its destination. For example, usable
marijuana for sale to a consumer must pass different tests than usable marijuana being sent to a
processor for extraction or concentration. For more information, go to
healthoregon.gov/marijuanatesting.
Products that fail their first state-required test may be reanalyzed and retested according to OHA rules.
If the initial test results in a failure, within 7 calendar days the producer or processor must request that
the original lab reanalyze the original sample, and the reanalysis must be completed by the lab within 30
calendar days of the request. If this reanalysis passes, within 7 days of the passing result the producer or
processor must request that a second lab resample from the original batch and a retest for the relevant
test(s). If the product passes both the reanalysis and the retest, the batch may be transferred to its
intended destination. If the product fails either the reanalysis or the retest, the batch must be either
remediated in accordance with OHA rules or destroyed in accordance with OLCC rules.
If you request a reanalysis or retest you must notify the OLCC of the request at the time it is made. See
the “Retesting and Remediation” section of this guide for more information.
The following guide explains how to use Metrc, the state’s Cannabis Tracking System, to record
sampling, testing, and remediation of recreational marijuana items.
DISCLAIMER: Over the course of 2022-2023 a number of new regulations and compliance tests will be
phased into effect, including the addition of mycotoxin, heavy metals, and microbiological contaminants
testing. See Appendix 2 for more details. This guide is meant to serve as an aid in explaining how to
correctly enter tracking data into Metrc. If you don’t understand this guide, please contact the OLCC for
help. This guide does not supersede any OLCC rule or OHA rule.

2
Definitions of Lots and Batches
OHA and OLCC rules specify the definition of a harvest and process lot. Specifically, a harvest lot is
defined as:
“a specifically identified quantity of marijuana that is cultivated utilizing the same growing
practices, harvested within a seven calendar-day period at the same location and cured
under uniform conditions.”
A process lot is defined as:
“(a) Any amount of cannabinoid concentrate or extract of the same type and processed using
the same extraction methods, standard operating procedures and batches from the same or
different harvest lot; or
(b) Any amount of cannabinoid products of the same type and processed using the same
ingredients, standard operating procedures and batches from the same or a different harvest
lot or process lots of cannabinoid concentrate or extract as defined in subsection (a) of this
section.”
Batch is defined as:
“(a) A quantity of usable marijuana from a harvest lot; or
(b) A quantity of cannabinoid concentrate or extract or cannabinoid product from a process lot.”
It is important to also recognize that a batch of concentrate or extract must be produced using a
standard operating procedure and result in one finished cannabinoid concentrate or extract that is
uniform in texture and form. A batch of an industrial hemp-derived vapor item must be made from a
standard operating procedure and result in one final industrial hemp-derived vapor item that is
uniform in flavor, texture, and form. Finally, a batch of a finished inhalable cannabinoid product must
be made from a standard operating procedure and result in one finished inhalable cannabinoid
product that is uniform in flavor, texture, and form.
When it comes to testing, batches, which are derived from harvest lots and process lots are what
“count” – that is, official sampling and lab testing by state-licensed and accredited labs must be done
at the batch level. See OHA rules on allowable batch sizes under OAR 333-007-0350 that may be
submitted for testing.
The quantity of sample material required to be taken from a batch and how it should be entered into
Metrc differs for usable marijuana as compared to extracts, concentrates, hemp vapor items and
cannabinoid products (e.g. edibles). The sections below describe the specific scenarios relevant for
the various product types.
Usable Marijuana Batch Testing
In the case of usable marijuana, a minimum of 0.5% of a batch will be sampled for testing. Under OHA’s
testing rules, samples of usable marijuana for potency testing must be strain-specific but can be
composited across batches; samples of usable marijuana for other testing (e.g. pesticides) may be
multi-strain but must be batch-specific. The figure below lays out the various permutations of how
batches can be tested and sampled. In this example, Acme Producer has two harvest lots.

3
•
The first harvest lot is comprised of Sour Diesel and Blue Dream plants that were harvested
within seven calendar-days of each other (October 1
st
to 8th). This harvest lot is a total of 50
lbs of finished (dried/cured) product.
•
The second harvest lot is comprised of Blue Dream and Jack Herer plants that were harvested
within seven calendar-days of each other (October 15
th
to 22nd). This harvest lot is a total of 45
lbs of finished (dried/cured) product
Although harvest lots, and therefore Metrc harvests, may be multi-strain, packages created out of
these harvests should be of a single strain. OLCC must be able to verify that testing is conducted in line
with rule – therefore, samples tested for potency must be drawn from single-strain packages.
Acme Producer has created five strain-specific packages out of the two harvest lots. Per ORELAP SOP,
sampling from multiple physical containers should be a two-step process. First, the requisite number of
increments should be taken (based on the product weight) and composited into a field sample. Second,
in the lab, a homogenized sub-sample should be created from the relevant field samples.
In line with ORELAP sampling protocols, in the below example of two harvest lots, a total of five field
samples would be taken (resulting in Samples 1 through 5 created as tagged Metrc sample packages).
Once back in the lab, two homogenized sub-samples would be created:
1)
Sample 6 sub-sampled from Samples 1 and 2 for water activity, moisture content, and pesticide
tests for Batch 1.
2)
Sample 7 subs-sampled from Samples 3 and 4 for potency test of Blue Dream strain split across
Batches 2 and 3.
The lab will now have seven samples in hand: Samples 1 through 5 that were taken (and tagged in
Metrc) in the field, and Samples 6 and 7 that were sub-sampled and homogenized (and tagged in Metrc)
in the lab.
Once these samples are created the test results will be entered as follows:
•
Sample 1 will be tested for potency; in Metrc this result will be tied back to Package 1 (Sour
Diesel);
•
Sample 2 will be tested for potency; in Metrc this result will be tied back to Package 2 (Blue
Dream);
•
Sample 6 will be tested for water activity, moisture content, and pesticides; in Metrc this result
will be tied back to both Packages 1 and 2 (the entirety of Batch 1);
•
Sample 3 will be tested for water activity, moisture content, and pesticides; in Metrc this result
will be tied back to Package 3 (Batch 2);
•
Sample 4 will be tested for water activity, moisture content, and pesticides; in Metrc this result
will be tied back to Package 4 (Batch 3);
•
Sample 7 will be tested for potency; in Metrc this result will be tied back to both Packages 3 and
4 (Batches 2 and 3);
•
Sample 5 will be tested for potency, water activity, moisture content, and pesticides; in Metrc
this result will be tied back to Package 5 (Batch 4).
Once all samples have had results entered into Metrc, the source packages will have had the full
spectrum of required tests conducted.
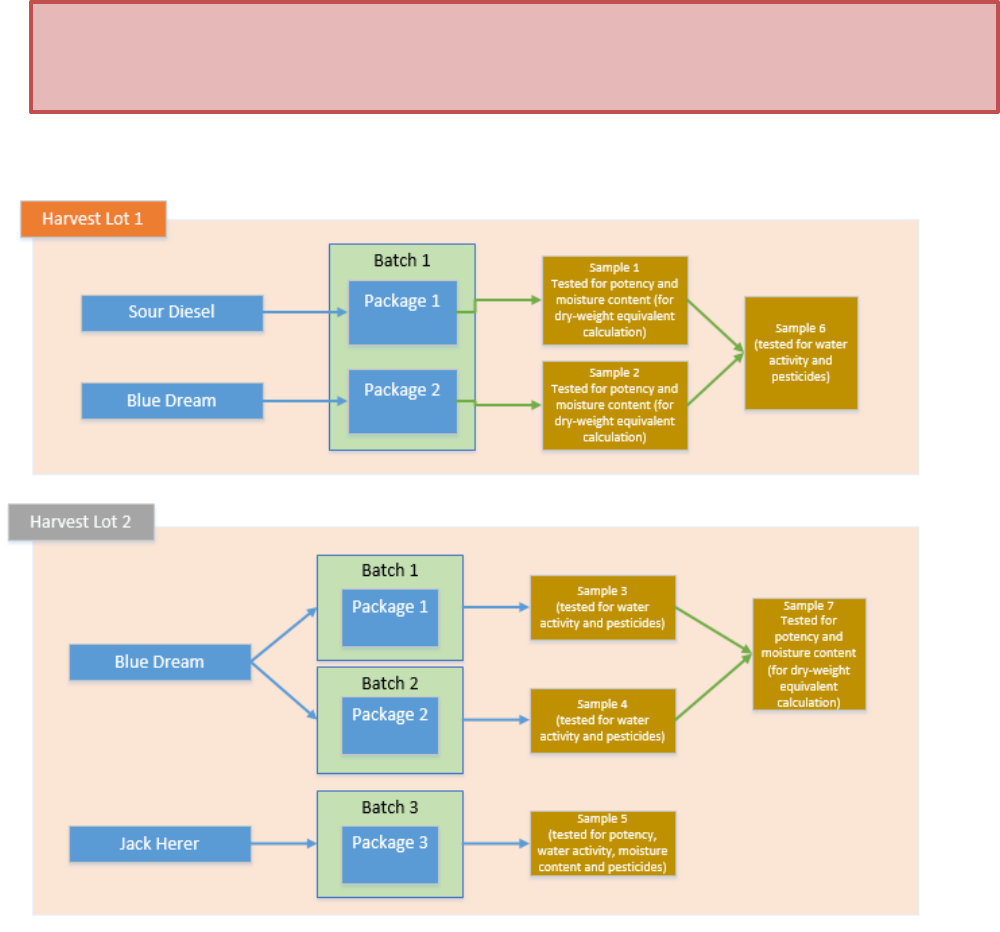
4
Figure 1a: Recommended Metrc Sampling Process, Usable Marijuana.
Metrc is configured to resolve its test status to either Passed or Failed only when all samples drawn
from a package have had a result entered. For example, Package 1 would only have a status of
“TestPassed” in Metrc when samples 1 and 6 all have results entered into Metrc. Because of this, it is
very important that the producer and lab creating the samples understand the rules and requirements
to ensure that testing is done properly and packages do not become “stuck” in a pending status.
There are rare occasions when licensees will request both kinds of composite testing across multiple
strains and batches. Figuire 1b below demonstrates an issue of Sample 4 not having any test results
applied to it. In that scenario the lab licensee should apply the test result “composite testing complete”
to that package so that metrc understands to finish changing the test status of the source packages.
DISCLAIMER: This figure does not display the Mycotoxin, Heavy Metals and Microbiological contaminant
tests which will be phased into the requirements in 2022-2023. However it does reflect the 50lbs batch
limit that begins July 1
st
2022.
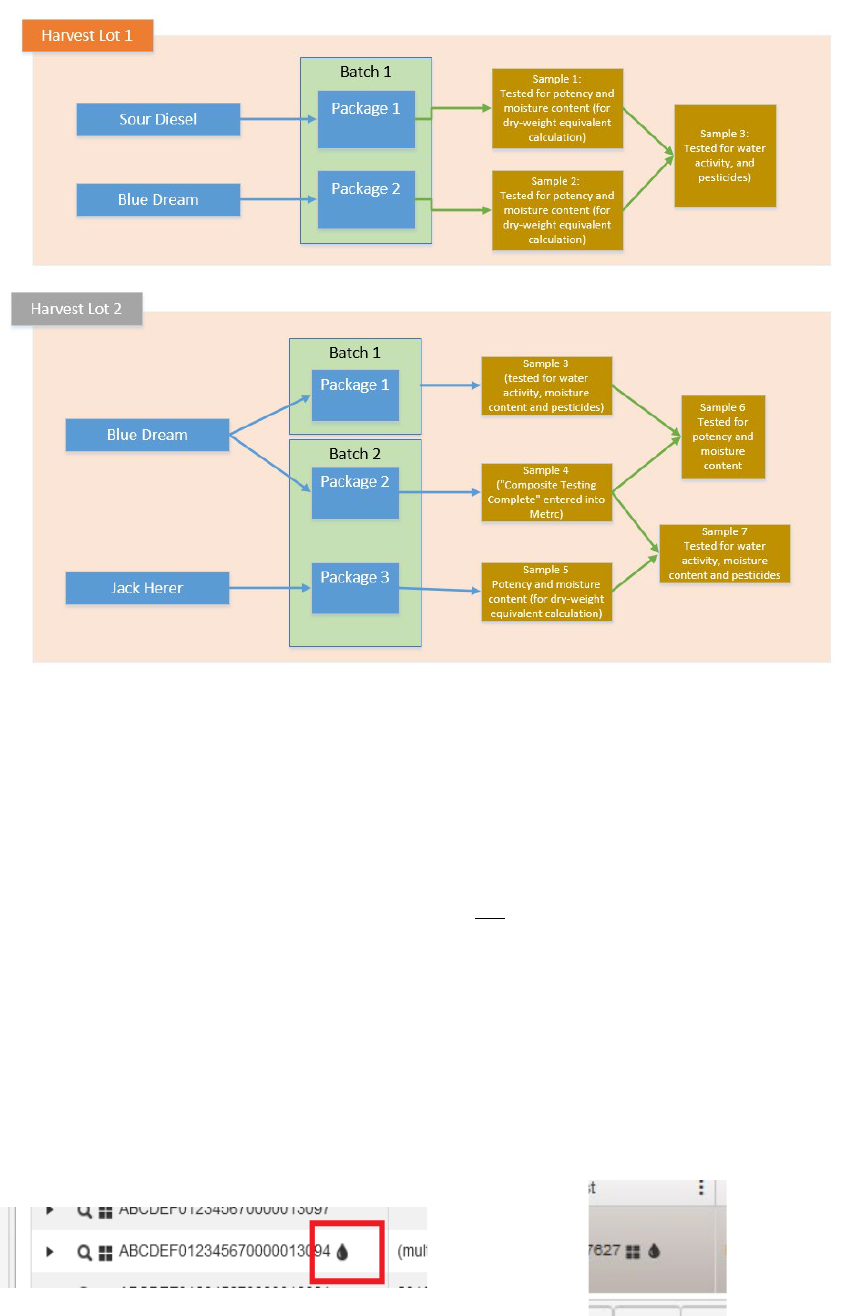
5
Figure 1b: “composite testing complete” test type
Important: composite samples (samples 6 and 7 in diagram above) should only be taken from sample
packages and not from “regular” packages. Taking a composite sample from a non-sample package will
not correctly link the test results to the source package being tested.
In Metrc, sample packages transmit results to the lowest-level non-sample package. If a composite
sample (e.g. sample 6 in diagram above) is pulled from a non-sample (“regular”) package (e.g. sample 1
and 2 in diagram above), the test results entered on the sample (sample 6) will be associated with the
immediate source of the sample (samples 1 and 2) but not the correct source package (e.g. packages 1
and 2 in the diagram above). In other words, if samples 1 and 2 were incorrectly created as “regular”
non-sample packages then Packages 1 and 2 would be missing results for water activity, moisture
content, and pesticides because those results would be “stuck” at the level of samples 1 and 2
Licensees are able to determine if a sample has been correctly made by looking for the correct icon in
both the Packages section of a licensee’s inventory as well as incoming manifests prior to receiving a
package.
Figure 2a: Sample Icon in Packages grid Figure 3b: Sample Icon in Incoming
Manifest grid

6
Extract, Concentrate, Finished Inhalable Cannabinoid Products, Cannabinoid Product and Industrial Hemp-
Derived Vapor Item Batch Testing
In the case of extracts, concentrate, and cannabinoid, the amount of material required for sampling
does not increase linearly. Instead, the sampling requirements “step up” in the number of sample
increments that must be taken according to the size of the process lot. Exhibit B, Table 7, of OHA’s
testing rules lay out the sampling requirements according to the batch/lot size in weight (for
concentrates/extracts/finished inhalable cannabinoid products and hemp vapor items). For example, a
0.5 lb process lot of extracts would require two samples which will be 1 primary and 1 duplicate. A 10 lb
process lot of the same product would require 5 samples increments which will be combined into
primary and 5 samples increments which will be combined into a field duplicate for a total of 10
samples increments.
Importantly, regardless of the number of increments required, only one Metrc sample package should
be created. The individual increments should be physically taken and kept separated by the lab as
required, but the aggregate weight of the total number of increments taken should be created in a
single Metrc sample package. The reason for this is twofold:
•
In the testing rules, the increments in total represent a single attempt at a pass or fail. If two of
four increments individually fail for pesticides, the batch as a whole has only one “strike” against
it. However, Metrc is designed to count each test failure of an individual test type as a “strike.”
Under the testing rules, two increments each failing should result in the batch being in a status
of “TestFailed” and eligible for reanalysis. If each increment were created as its own Metrc
sample package, though, the failures of the two individual increments would be counted twice
and result in a status of “RetestFailed,” which would incorrectly make it ineligible for
reanalysis/retesting.
•
If each increment has test results entered individually the test results are significantly more
difficult to decipher. Large process lots may have many individual increments sampled and
tested. By consolidating all testing information under a single Metrc sample package it makes
it clearer what information should go on the label and whether a batch passed or failed
testing.
The figure below lays out the various permutations of how batches from process lots can be tested and
sampled based on the product type.
In this example, Omega Processor has two process lots:
•
3.5 lbs of Extract oil
•
24 units of truffles
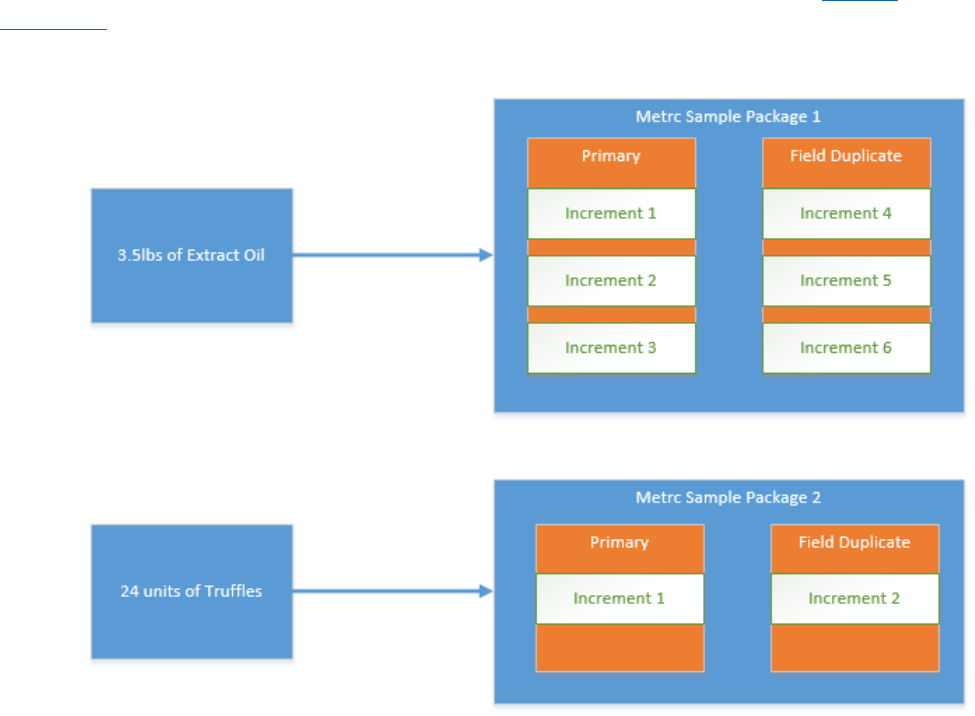
7
Omega Processor has hired Alpha Labs to conduct testing on each of the process lots. The sampling and
testing procedure is as follows:
•
Six increments will be taken from the 3.5 lbs process lot of oil. A single Metrc sample package
will be taken that accounts for the aggregate weight of the individual increments. Three
increments will be combined to make the primary and three increments will be combined to
make the field duplicate sample. The primary and duplicate will be tested individually and then a
single set of test results will be entered into Metrc.
•
Two increments will be taken from the 24 unit process lot of truffles. A single Metrc sample
package will be taken that accounts for the aggregate weight of the individual increments. One
of the increments will be used for a primary and the second increment will be used for a field
duplicate. The primary and field duplicate will be tested individually and then a single set of test
results will be entered into Metrc.
For more information regarding how the single set of test results should be entered see the Entering
Test Results section of this guide.
Figure 4: Recommended Metrc Sampling Process;
8
Identifying Lots and Batches
Lots and batches each must be stored and labeled in a certain way per OLCC and OHA rules. According
to OLCC rule, within 45 days the harvest lot must be physically separated from other lots “in a receptacle
or multiple receptacles” with each container having a RFID tag attached. This physical separation and
identification with a RFID tag is accomplished by creating packages out of Metrc harvests, and within the
system you will select the ID of the tag that will be physically affixed to the receptacle(s) containing the
harvest lot material.
Batches must also be stored and labeled in a certain way under OAR 845-025-5720. Batches with
samples taken and awaiting test results must be labeled with:
•
The licensee’s license number;
•
The harvest or process lot unique identification number;
•
The name and accreditation number of the laboratory that took samples and the name and
accreditation number of the laboratory that will perform the testing, if different;
•
The test batch or sample unique identification numbers supplied by the laboratory personnel;
•
The date the samples were taken; and
•
In bold, capital letters, no smaller than 12 point font, “PRODUCT NOT TESTED.”
The batch must also be stored in a way that:
•
prevents the product from being tampered with or transferred or sold prior to test results being
reported and
•
makes the batch able to be easily located and identified for the Commission or laboratory upon
request.
Importantly, under rule a batch may be stored in more than one receptacle as long as the labeling and
storage requirements are met. In practice that means that multiple Metrc packages may be considered
as part of the same batch as long as all other requirements are met. Packages 1 and 2 in the example
above fit those criteria – they are from the same harvest lot and together do not exceed 15 pounds.
Within Metrc, it is packages that are submitted for testing. It is the responsibility of the producer or
processor to ensure that the Metrc packages being submitted for testing meet the requirements and
definitions of batches as laid out in Oregon administrative rule. That means that any package of usable
marijuana must be verifiably less than 50 pounds within your Metrc inventory at the time it is submitted
for testing.
9
Creating Samples
To meet testing requirements, physical sampling from a batch must be done by the employee of an
OLCC-licensed and ORELAP-accredited laboratory. The physical material sampled must then be reflected
in the inventory of the licensee who currently holds the source being sampled from.
In Metrc, samples are taken from packages.
2
The final test results will not be registered until all sample
packages taken from a single harvest or package have been tested – if there are additional sample
packages without a test result, the test status for the source package or harvest will remain
“TestingInProgress.”
Sampling in Metrc is done by selecting the package and then clicking the “Submit for Testing” button.
According to ORELAP’s sampling protocol, physical samples should be “randomly selected from different
locations within a container or set of containers.” In practice, the producer or processor will identify for
the sampler which container or set of containers constitute the batch to be submitted together for
testing. The sampler will then physically take samples from each container as part of the batch in line
with ORELAP sampling protocols. If the sampling is done at the site of the producer or processor, once
the samples are physically taken, then the sampler should instruct the licensee how much weight the
sample package(s) should contain from each individual container. If the sampling is being done on the
lab’s premises, the lab will create the sample package in their own Metrc account.
For more information regarding sampling procedures in Metrc, see the Usable Marijuana Batch Testing
section of this guide.
To create a sample package, follow the below steps:
1)
Select the source of the sample (the Metrc package from which the sample is being taken). If
you are sampling from multiple packages, highlight each of the packages that will be submitted
for testing together under the same sample.
Figure 5: Sampling from a Metrc package
2
In Metrc, there are two types of packages – a “regular” package and a sample package. “Regular” packages are those that are
created through the Create Packages button in Metrc. Sample packages are those that are created through the Submit for
Testing button. The final test results will not be registered until all sample packages taken from a single “regular” package have
been tested – if there are additional sample packages without a test result, the test status for the source package will remain
“TestingInProgress.”
Click and highlight
the package you will
be sampling from
Select Packages and
click on the Active tab
10
2)
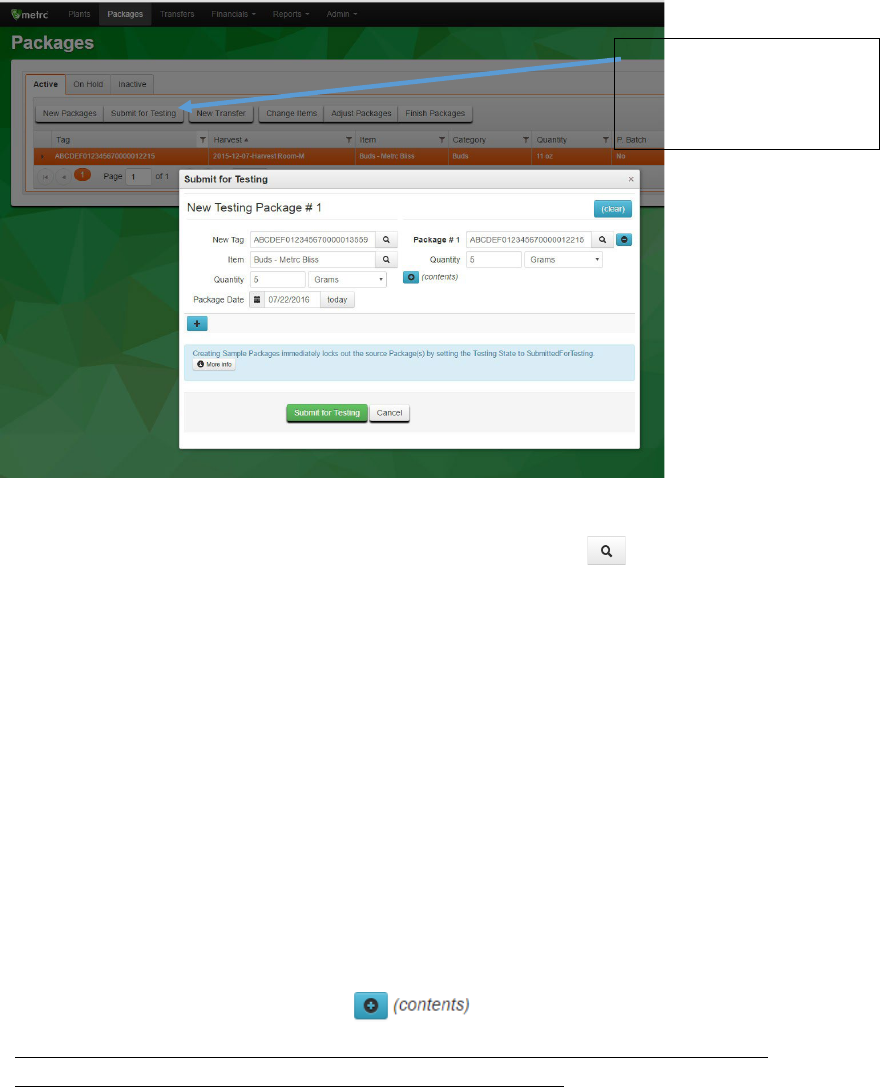
Click to highlight the package, then click the Submit for Testing button and complete the fields in
the box that displays.
Figure 6: Complete the Submit for Testing fields
New Tag: This is the RFID tag to be associated with the new sample package. Either type the
last few numbers of a tag and select the correct one or click the lookup button and
search for the tag
Item: This is the item of the sample package. It should match the item of the package from
which the sample is being taken. For example if the item of the source is “Buds – Metrc
Bliss,” this would also be the item of the sample package.
Quantity (on the left-hand side): This is the quantity of the sample package (either count or
weight of the contents).
Package Date: This is the date when the sample package was created.
Package #1: This is the RFID tag of the source package.
Quantity (on the right-hand side): This is the quantity being sampled from the source
package.
If there are additional source packages being sampled from that should be part of the same
sample package, you can click on the button and add additional sources.
Please note: all test results from this sample package will be associated with every
source package you include in the sample package contents.
3)
When all the information for your sample package has been created, click on the Submit for
Testing button. Once submitted, the sample package should appear in the Packages inventory
Click the Submit for
Testing button and fill out
the fields that appear
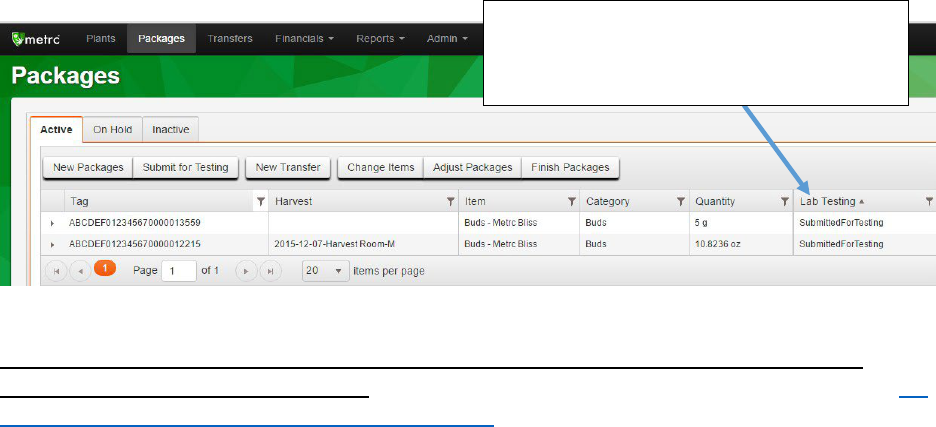
11
and the source package(s) and the sample package will now have the status of
“SubmittedforTesting.” The weight of the source package will be automatically updated to
reflect the weight taken out for sampling.
Figure 7: Sample package appears in package inventory
Important: incorrectly creating a non-sample (“regular”) package can prevent test results from
correctly being tied to a source package. This is especially true in the case of composite testing; see the
section in this guide on Usable Marijuana Batch Testing for more information.
Sample package will appear in inventory
and both source package and sample
package will now say “SubmittedforTesting”
12
Entering Test Results
Only labs have the ability to enter test results into Metrc. Each set of test results should only be entered
into Metrc once per sample. In the case of a reanalysis or retest see the Retesting and Remediation
section of this guide. At the time that all testing has concluded and the final results are known for the
full battery of tests that have been run, those results will all be entered in the same place. Please note
that the final test results will not be registered until all sample packages taken from a source package or
harvest have been tested – if there are additional sample packages without a test result, the test status
for the source package or harvest will remain “TestingInProgress” even after test results have been
entered.
Test results should only be recorded for test types found in the testing rules. There are five categories
of testing that must be entered into Metrc if conducted: pesticides, solvents, water activity and
moisture content, microbiological contamination, and potency (THC and CBD). Refer to the Oregon
Health Authority rules on cannabis testing
for the full explanation of when each test is required and
what constitutes passage or failure for each. For an explanation of how to handle non-required testing
(e.g. testing for terpenes), see the FAQ in Appendix 1. For an explanation of how to handle Research and
Development testing, see the
Research and Development Testing section of this guide.
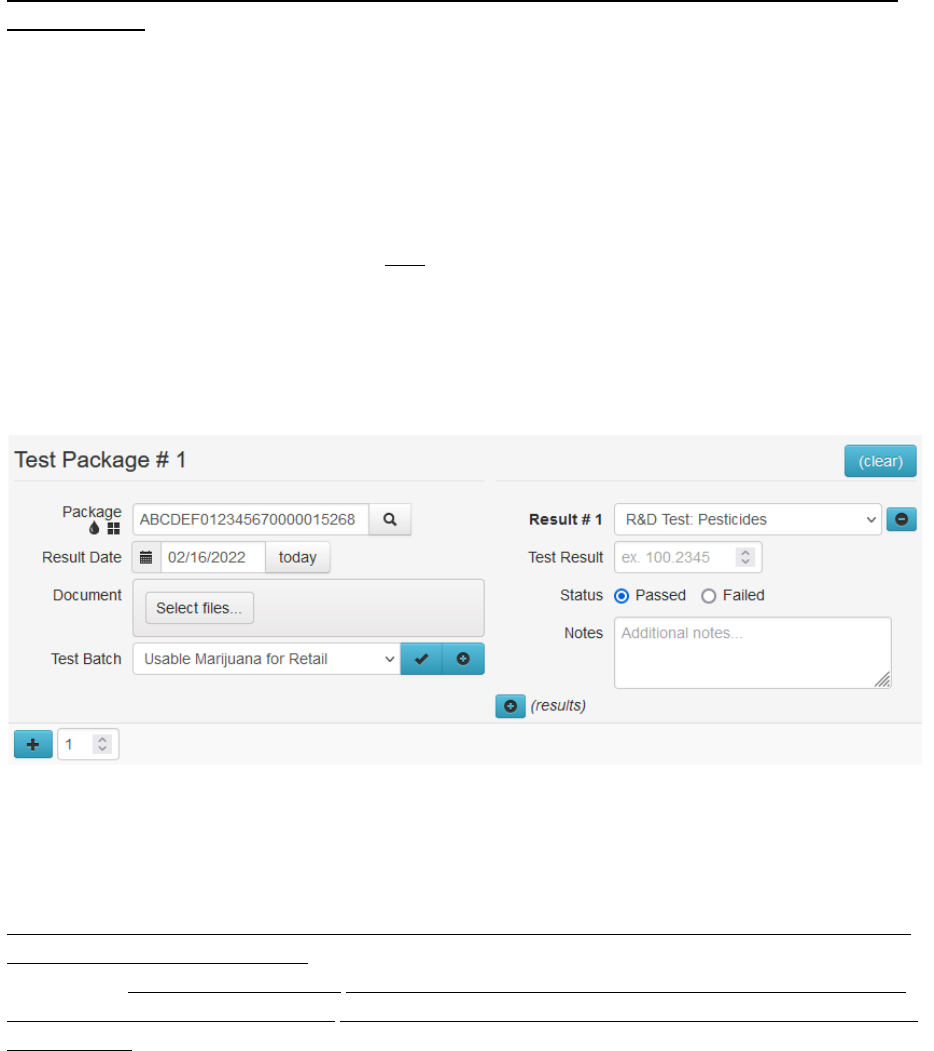
To enter test results into Metrc, follow the below steps:
1)
In your Metrc lab facility account in the Packages section, click the Active tab. Select the sample
package being tested and click the Test Packages button.
Figure 8: Select sample package for testing
2)
Complete the fields in the Test Packages box.
Package: RFID tag of the sample package being tested.
Results Date: The date the test result for the sample package is being reported after all
tests have been completed. Please note: this may not be the date that an individual
test has been run.
Result #1: The test type being run.
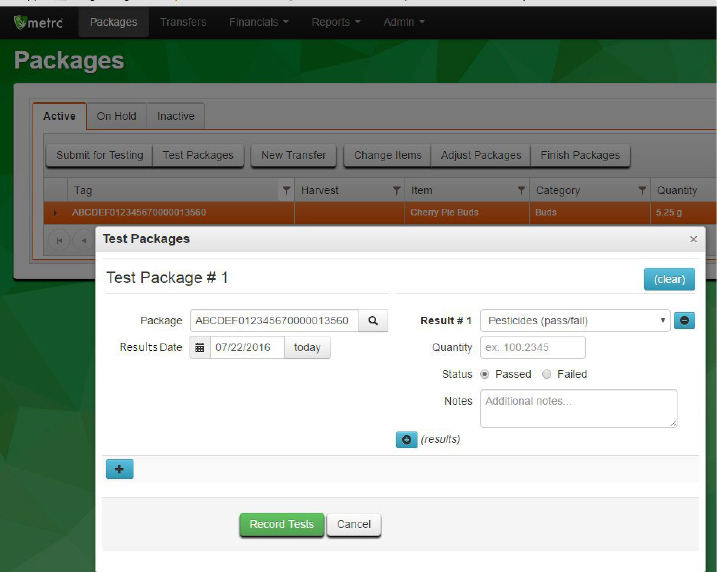
13
Quantity (may be optional or required depending on test type): The test value of the
individual test being run. This should be reported in the unit in parentheses in the test
type name. For example, “Abamectin (ppm)” should be reported as parts per million,
whereas “Total THC (mg/g)” should be reported as milligrams of THC per gram of
sample.
Status: Whether the individual test passed or failed.
Notes (optional field): A place for notes, such as a LIMS record number, comments to
the producer or processor requesting the tests, etc.
In the case of pesticide testing, if no analyte is detected above the level of quantification then
only “Pesticides (pass/fail)” must be entered (with no quantity). However, if an analyte is
detected above the level of quantification, the specific value detected must be entered – even if
it is below the action limit. In this case “Pesticides (pass/fail)” and the individual analyte would
both be marked as “pass” and the analyte would have a specific detected quantity entered. See
the screenshots below for an example and Appendix 4 for more detail on how to enter test
results for pesticides and other test categories.
Figure 9: Reporting a passing pesticide result with no analytes detected
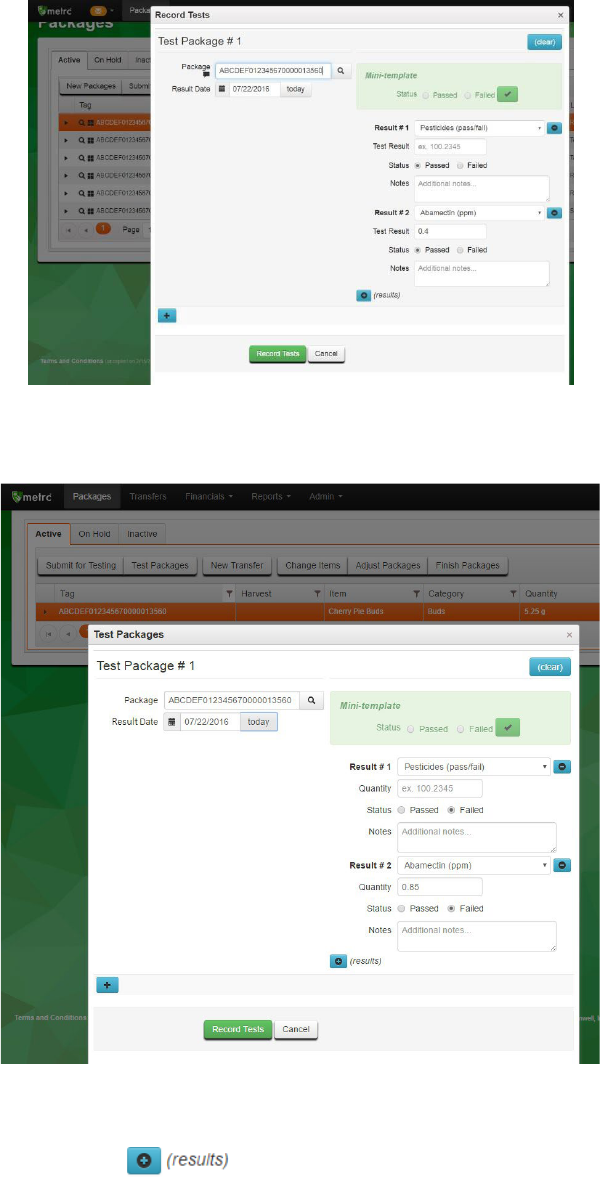
14
Figure 10: Reporting a passing pesticide result with one analyte detected below action limit
Figure 11: Reporting a failing pesticide result
3)
Enter results for each test being conducted on the sample package. You can enter additional
results by clicking on the button. Once all tests have been entered, click the Record
Tests button.

15
Entering results for extract, concentrate, finished inhalable cannabinoid products, cannabinoid products, and
industrial hemp-derived vapor items increments
Although multiple increments are sampled and tested for extracts, concentrates, and cannabinoid
products, as described in the above explanation of sampling protocols for such products,
a single result
should be entered into Metrc for the collection of increments.
There are two ways that extracts, concentrates, and cannabino
id products can fail compliance testing:
having levels that are too variable or having levels that exceed the allowed for threshold. The way
results should be entered will depend on the way in which the sampled process lot passed or failed.
•
All increments pass (variability within allowed range and all levels below action limits): lab
should enter the RPD/RSD between increments (enter value and mark as passed) and the
weighted average value for all required tests.
Example: An extract intended for sale at retail is tested for solvents, pesticides and
potency. No pesticide analytes are detected, THC levels for all increments are below
limits, and RPD between increments is less than 10%. In Metrc the lab would enter:
o
“Pesticide (pass/fail)”: pass; no value required
o
“Potency (pass/fail)”: pass; no value required
o
“Total THC (mg/g)”: pass; enter average Total THC of increments tested (this is the
value that should be placed on the label when sold to consumers)
o
“Total CBD (mg/g; cannot fail)”: pass; enter average Total CBD of increments
tested (this is the value that should be placed on the label when sold to
consumers)
o
“THC (%RPD)”: pass; enter RPD detected between increments
•
Variability between increments too high but all levels below action limit: lab should enter the
RPD/RSD between increments (enter value and mark as failed) and the weighted average value
detected for all required tests.
Example: A concentrate intended for sale at retail that has passed control study testing
is tested for pesticides and potency. No pesticide analytes are detected and THC levels
for all increments are below limits, but RPD between primary and field duplicate
exceeds 10%. In Metrc the lab would enter:
o
“Pesticide (pass/fail)”: pass; no value required
o
“Potency (pass/fail)”: pass; no value required
o
“Total THC (mg/g)”: pass; enter average Total THC of primary/field duplicate (this
is the value that should be placed on the label when sold to consumers)
o
“Total CBD (mg/g; cannot fail)”: pass; enter average Total CBD of primary/field
duplicate (this is the value that should be placed on the label when sold to
consumers)
o
“THC (RPD)”: fail; enter RPD detected between primary and field duplicate
•
One or more levels above action limit: for failing test type(s) enter maximum value detected;
for passing test type(s) enter weighted average value detected; enter RPD/RSD between
increments as passing or failing (whichever applies).
16
Example: An edible intended for sale at retail that has passed control study testing is
tested for potency. RPD between the primary and field duplicate is less than 10% but
the THC within the “recreational grade” unit for sale exceeds the concentration limit. In
Metrc the lab would enter:
o
“Potency (pass/fail)”: pass; no value required
o
“Total THC (mg/g)”: fail; enter maximum Total THC detected
o
“Total CBD (mg/g; cannot fail)”: pass; enter average Total CBD of primary/field
duplicate
o
“THC (RPD)”: pass; enter RPD detected between primary and field duplicate
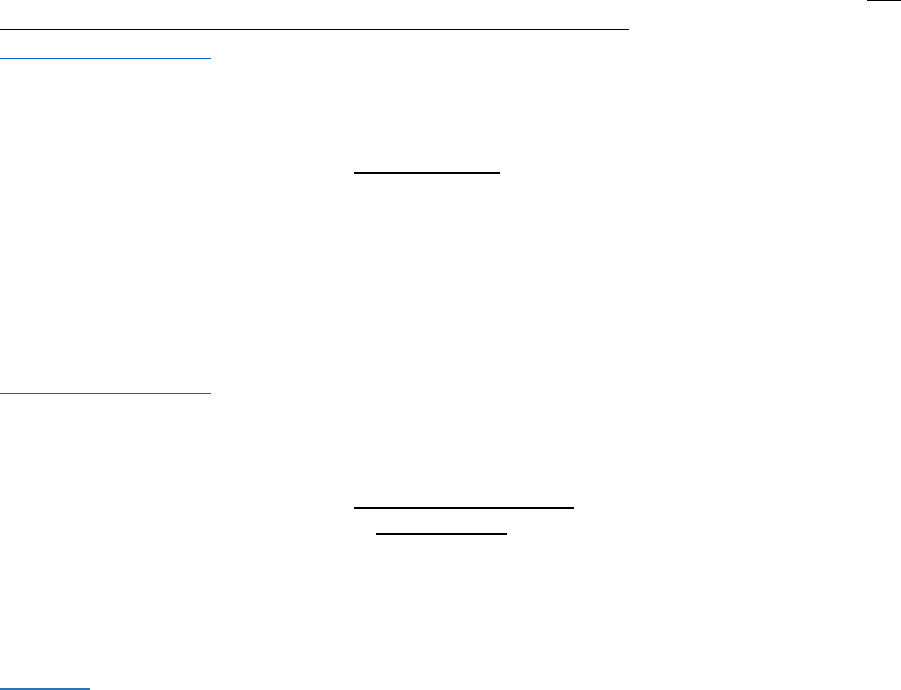
17
Retesting and Remediation
If a batch passes all state-mandated testing, it can be transferred to its intended destination. If,
however, the batch fails any of the state-required tests, the originating licensee has two options,
depending on the test type that resulted in the failure. First, regardless of test type, the licensee may
request a reanalysis and retest be conducted. Second, for all tests other than pesticides, the licensee
may remediate the product. Remediation may occur after the first failure on the batch or if the retest
process results in a second failure.
If a licensee’s product fails a test and the licensee wishes to initiate reanalysis of the sample by the
original lab, the licensee must request the reanalysis from the original lab within 7 calendar days and
must notify the OLCC of this request at the time the request is made. To notify the OLCC, email
[email protected] with the subject line of “Request for Reanalysis” including the requesting
licensee’s full 14 digit license number (e.g. “Request for Reanalysis 020-10000000123”) and include the
following information:
a.
The requesting licensee’s full 14 digit license number and facility name (as they appear in Metrc)
b.
The Metrc package tag ID of the sample package being reanalyzed
c.
The lab doing the reanalysis
d.
Date of request for reanalysis
e.
Test(s) requested for reanalysis
If the reanalysis results in a passing test the licensee must request a resampling and retesting by a
second lab within 7 calendar days of receiving the reanalysis test results from the first lab. The licensee
must also notify the OLCC of this request for retesting at the time it is made. To notify the OLCC, email
with the subject line of “Request for Retesting” including the requesting
licensee’s full 14 digit license number (e.g. “Request for Retesting 020-10000000123”) and include the
following information:
a.
The requesting licensee’s full 14 digit license number and facility name (as they appear in Metrc)
b.
The Metrc package tag ID of the original sample package that was reanalyzed
c.
The Metrc package tag ID(s) of the batch package that will be retested
d.
The lab doing the retesting
e.
Date of request for retesting
f.
Test(s) requested for retesting
Retesting
To pass retesting a batch must be tested twice for the original test that failed, and both of these must
result in a passing result. The lab that conducted the original testing will rerun the reanalysis on the
original sample material. In Metrc, a passing result at this stage will result in the batch lab status
becoming “AwaitingConfirmation.”At that point a second lab will resample from the batch and conduct
a retest. If the retest passes then the batch’s lab status in Metrc will become “RetestPassed.” If either of
these retests results in a failure, however, the status will become “RetestFailed.” A package with a status
of RetestFailed has reached a testing “dead end” and must be either remediated or destroyed.
To illustrate the retesting process, assume that a batch of processed oil is intended to be transferred to
a processor for further processing. Alpha Labs conducts testing and although the sample passes testing
for potency and pesticides, it fails for residual solvent because the analyte Benzene was detected at a
18
level above the action level. In Metrc, the batch now has a status of TestFailed. The processor requests a
reanalysis, at which point Alpha Labs reanalyzes for Benzene and finds a passing result. The batch’s
status in Metrc will now become AwaitingConfirmation. Subsequently, Beta Labs takes a sample from
the original batch – a sample is created in the producer’s inventory as described in the “Creating
Samples” section of this guide by submitting the package for testing. The sample is manifested to Beta
Labs, and once accepted into Beta Lab’s inventory a test for Benzene is conducted. Beta Labs confirms
the passing result for Benzene. The batch’s status becomes R
etestPassed. The batch has now passed
testing and may be transferred for further processing.
If a producer or processor requests retesting, no special considerations need to be made in Metrc. As
long as the specific test(s) that failed are submitted with passing results in Metrc, the system is
configured to automatically carry the batch through the various stages of the retesting process.
Importantly, Metrc does this on a test type basis for all tests that were entered with results of “failed” in
the first round of tests. In other words, if the original lab submitted failing results for both “Solvents
(pass/fail) and “Benzene (µg/g),” the retests must submit results for both of those test types (see Figure
12).
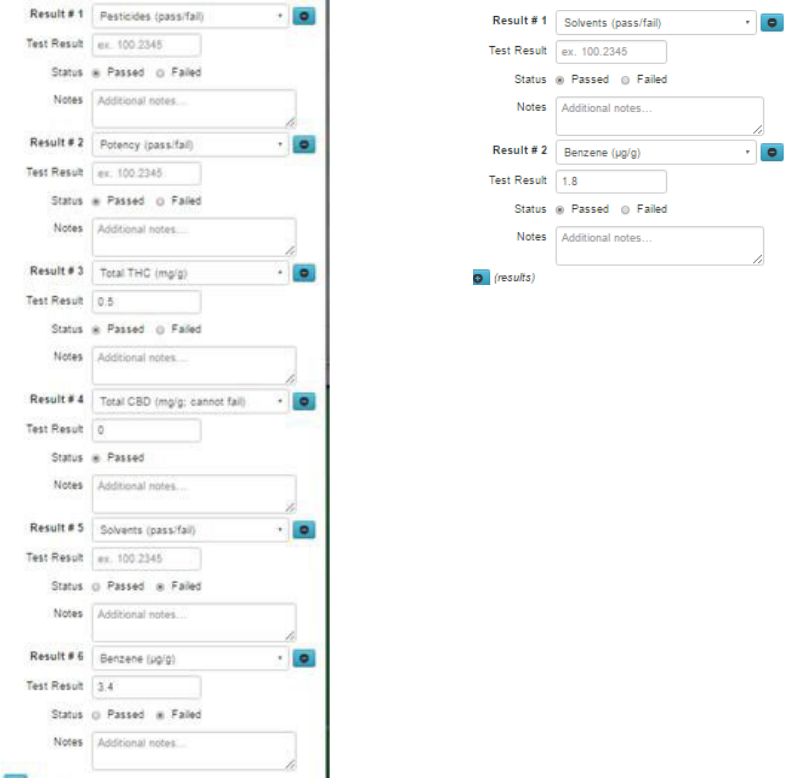
19
Figure 12: Original results (left) and Retest results (right)
20
Remediation
In cases allowed for under rule, remediation is a second option available to a licensee in the result of a
test failure (either after one failure or if a retest results in a failure). Producers cannot remediate for
pesticides under rule, and a batch of usable marijuana failing for pesticides must be destroyed.
3
In the
case of extracts and concentrates, if 100% of the usable marijuana used as an input in the process lot
was tested for pesticides by a licensed and accredited lab and the test results for the pesticide results
are in Metrc then the failed process lot may be remediated in a way that decreases the pesticide
concentration below the action limit. Following remediation the process lot must be resampled and
retested; if the lot fails after remediation it must be destroyed. Refer to OHA’s testing rules for complete
information regarding proper remediation.
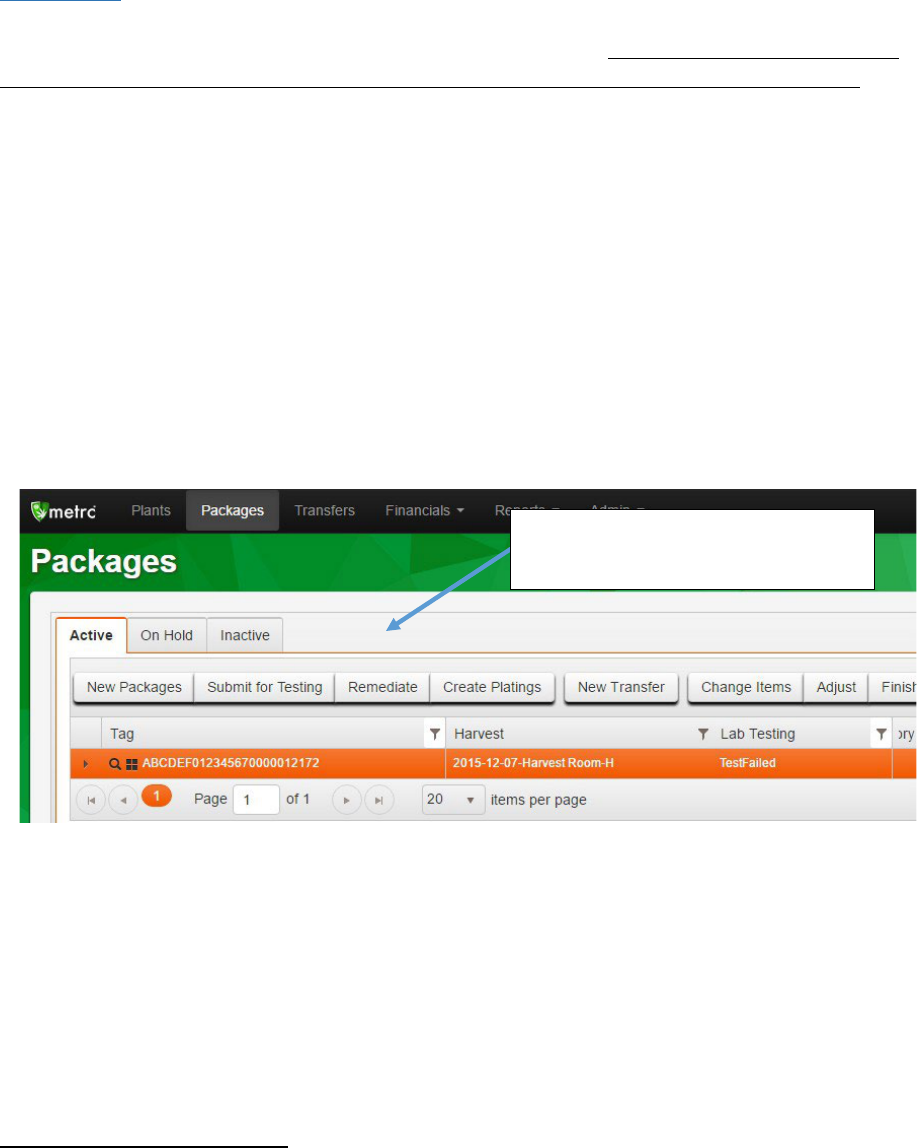
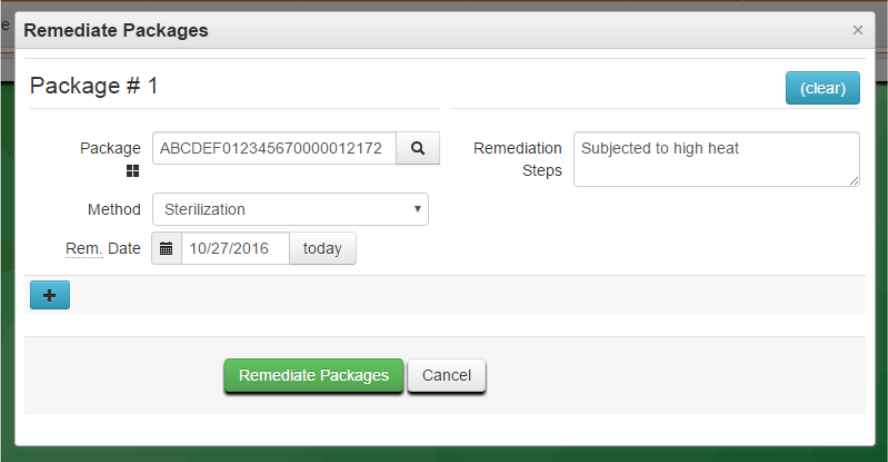
In Metrc, remediation must be done on the source package by clicking on the “Remediate” button in the
packages tab. In the “Remediate Packages” box you must select the Method and Date of Remediation as
well as detail the steps taken to remediate the package. Once the fields are completed, click submit, and
the package lab status will now be “Remediated.” If the batch requires retesting following remediation,
you will need to resubmit for testing as described in OHA’s testing rules. See Appendix 3 for more detail.
Figure 13: Remediating a package
3
If a sample from a batch of usable marijuana fails pesticide testing but only for the analytes piperonyl butoxide or pyrethrins,
and the Oregon Department of Agriculture determines that the products used were listed on the Department’s Guidelist for
Pesticides and Cannabis and the product was applied in accordance with the label, the OLCC may permit the producer or
grower to remediate the usable marijuana using procedures that would reduce the concentration of pesticides to less than the
action level. Remediation cannot occur unless OLCC permission has been granted.
Highlight the relevant package and
click the “Remediate” button
21
Figure 14: Remediating through Sterilization
22
Research and Development Testing
The OHA’s testing rules distinguish between Compliance Tests and “Research and Development” tests.
Importantly, all products must undergo all required compliance tests prior to being transferred to
other licensees; research and development tests can be done for a licensee’s own quality control and/or
R&D but do not “count” as a pass for state-mandated testing. Although the sampling and testing
protocols are not as stringent for Research and Development testing, the following requirements apply:
•
All transfers between licensees must be tracked in Metrc, and
•
Usable marijuana is not eligible for Pesticide Research and Development testing.
The licensee should create a “sample” package and transfer it to the lab. There is a “R&D test:” option for
each type of R&D test in Metrc. The lab should select the correct R&D test and enter the results of the
R&D test. A certificate of analysis (COA) is NOT required to be entered into Metrc for an R&D test. Test
quantities (e.g. pesticide parts per million or THC/CBD percentages) are not required to be entered for
R&D tests, but the test should be marked as passed or failed based on the relevant compliance test action
and/or concentration limit.
Figure 15: R&D Test sample Input
Once the sample has been given either a “Passed” or “Failed” result its test status will revert to “Not
submitted.”
R&D alone tests are NOT sufficient to meet the testing requirements if the package has not passed
for all required compliance tests. It is the licensee’s responsibility to understand and comply with the
testing rules. Transferring product to another licensee that has not passed all required compliance
testing could result in an OLCC rules compliance violation and affect your ability to work or operate
your business

23
Tentatively Identified Compounds (TICs)
According to OHA’s testing rules, in addition to the state-required test results that must be submitted in
Metrc, accredited labs may be required to notify the appropriate Agency (OHA or OLCC) of Tentatively
Identified Compounds (TICs) that have the greatest concentration in the material being tested. The
definition and method of detection of TICs can be found in OHA’s rules
regarding testing and
accreditation of labs.
If TICs are required, the lab should select the lab test type of “Tentatively Identified Compound (cannot
fail)” and select “Passed” as the Status. In the notes field the lab should then identify the individual TICs
detected, as well as any information regarding each TIC per OHA’s testing rules.
Figure 16: Reporting Tentatively Identified Compounds to OLCC
24
Reconciling Weight Taken for Testing (Adjustments)
Labs, like other licensees, must reconcile their physical inventory in Metrc. Any products receive from or
transferred to other licenses must be recorded in Metrc at the time of transfer and receipt. However, in
the case of subsampling for the purposes of testing, labs only need to reconcile inventory when all
testing is complete and results are entered into Metrc. The adjustment at the end of the testing period
must detail why each portion of the sample is being destroyed.
For example, consider a lab that has a sample package of 20 grams. Over the course of the week the lab
conducts 5 tests using 1 gram of material each, then destroys the remainder of the sample material.
During the week, 5 grams have been lost due to moisture loss/evaporation. In this example at the end of
the week the lab would submit three adjustments:
1)
-5 grams adjusted with a reason of “Sample Destroyed (Lab Use Only)”;
2)
-10 grams adjusted with a reason of “Waste”; and
3)
-5 grams adjusted with a reason of “Moisture Loss.”
The process for inventory reconciliation is done via package adjustments, and there are two ways to do
this within Metrc.
1)
Via the Packages tab
a.
In your lab facility account, click on the Packages tab.
b.
Highlight the package(s) to be adjusted and click the Adjust Packages button.
c.
In the “Adjust Packages” box, fill out the relevant fields.
Package: RFID tag of the package being adjusted.
Quantity +/-: The amount of weight to adjust the package by. Important: if subtracting
weight from a package, the quantity must have a minus sign (-) before the number.
Adj. Date: The date the adjustment was made.
Reason: Reason the adjustment is being made. Adjustments due to testing should use
the “Sample Destroyed (Lab Use Only)” reason code.
Note: Note to indicate reason for adjustment, tests done, etc. – optional field for
reference only
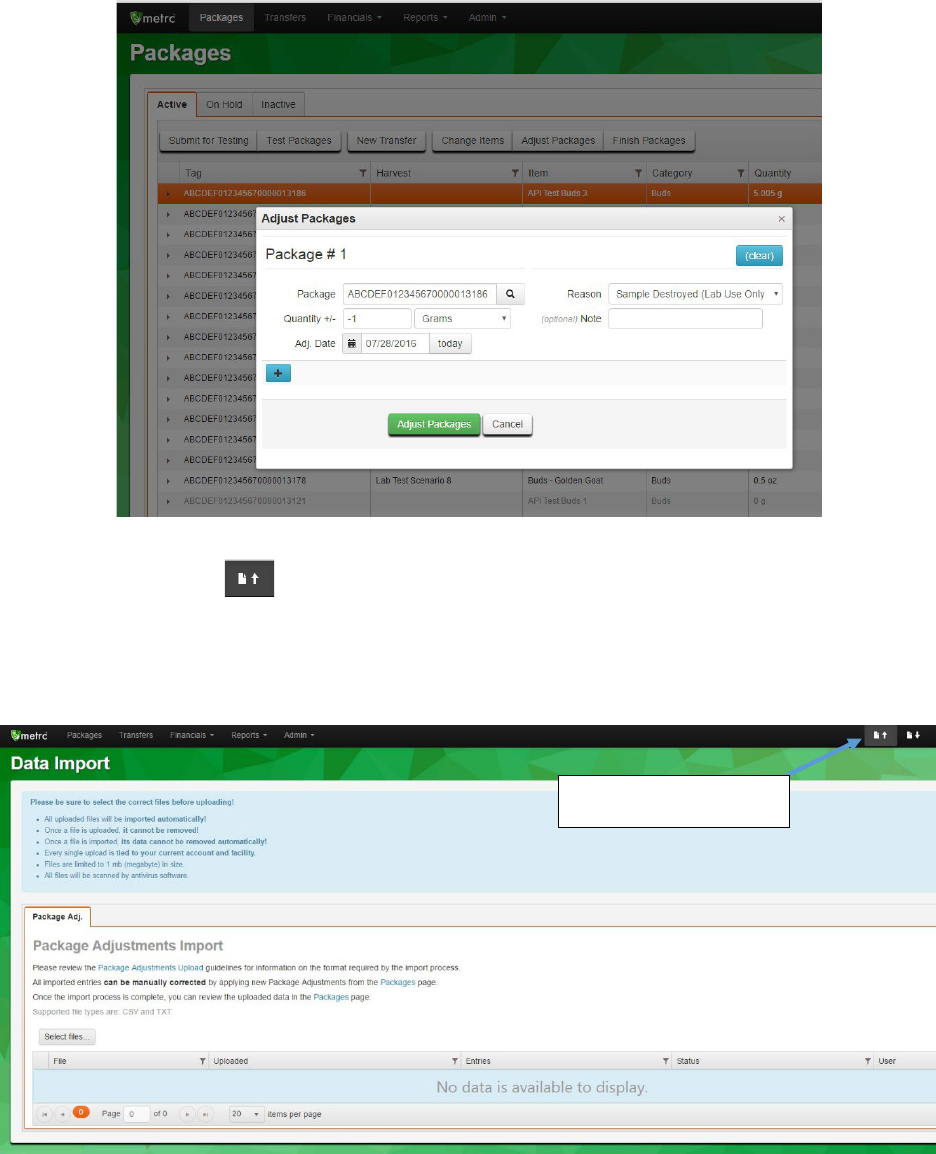
25
Figure 17: Adjustments through the Packages tab
2)
Via the Data Import function
a.
Click the button on the top right of the screen.
b.
Review the Package Adjustments Upload instructions, select the CSV or TXT file you wish
to upload, and upload the file.
Figure 18: Adjustments via Data Import
File Upload button

26
Lab Subcontracting
If a lab is not accredited for the full scope of state-required tests they will need to subcontract to
another OLCC-licensed lab that is ORELAP-accredited for the relevant tests needed. For example, if a lab
is not accredited for pesticide testing, a second lab would need to be subcontracted to conduct those
tests before a product could be transferred to a retailer for sale to a consumer.
According to ORELAP guidance, the primary testing lab will submit all test results into Metrc for a batch,
even if a subcontracted lab conducted tests. However, because OLCC requires that all marijuana items
transferred between licensees be documented in Metrc, subsamples sent to a subcontracted lab must
be recorded in Metrc like any other sample or transfer. There are several different types of
subcontracted test types available to the subcontracted lab to choose from, based on the type of
subcontracted test the lab is conducting. The subcontracted test types are:
- Subcontracted test (Potency)
- Subcontracted test (Pesticides)
- Subcontracted test (solvents)
- Subcontracted test (water activity)
- Subcontracted test (moisture content)
The following steps should be taken by both the primary and secondary lab:
1)
The primary lab should submit the primary sample for testing in order to create a subsample.
This requires the lab to have RFID package tags in stock. Refer to the “Creating Samples” section
of this guide.
2)
The primary lab will create a transfer manifest to the subcontracted lab.
3)
The subcontracted lab will accept the transfer into their inventory. Outside of Metrc tests will
be conducted on this sample and results will be communicated to the primary lab. The
subcontracted lab does not need to upload their Certificate of Analysis into Metrc. The
primary lab will upload the Certificate of Analysis.
4)
The subcontracted lab will choose the subsample package in Metrc and select “Test Package.”
5)
The subcontracted lab will select the “Subcontracted test” test type that matches the test
being performed, for example “Subcontracted test (Pesticides)”. A “passing” result for
“Subcontracted test” is entered regardless of the actual test performed or the actual result
(pass/fail) and simply indicates that the test was conducted. The primary lab will enter the
actual certified pass/fail result of any subcontracted tests conducted.
6)
Once the subcontracted lab’s results have been communicated to the primary lab and results
certified, the primary lab should submit all test results for the batch into Metrc and upload
the Certificate of Analysis into M.
For example, Alpha Labs samples a batch of usable marijuana that is intended for retail. However, Alpha
Labs is not accredited for pesticide testing and must subcontract with Beta Labs. Alpha Labs creates a
subsample to transfer to Beta Labs. Beta Labs conducts the pesticide results and, outside of Metrc,
provides the full set of results and whether pesticides passed or failed. Within Metrc Beta Labs selects
the subsample in their inventory, clicks the “Test Package” button, and submits a result for
“Subcontracted test (Pesticides)”.
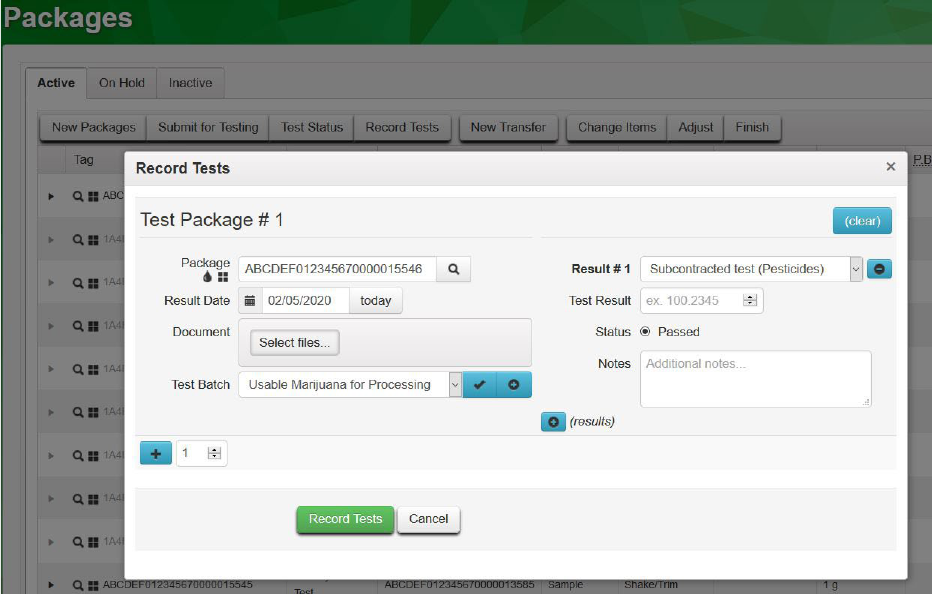
27
Figure 19: Subcontracted Lab Documenting Test
28
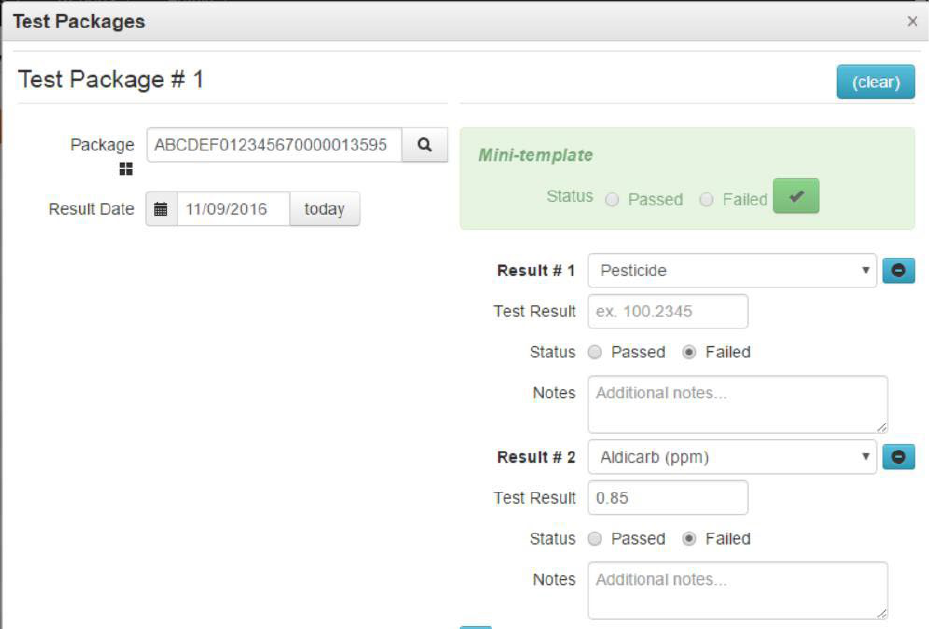
After Alpha Labs receives and certifies the full test results from Beta Labs they will enter the specific
tests conducted and results. See the “Entering Test Results” section of this guide and Appendix 4 for
more information. In this example, Beta Labs detects the analyte of Aldicarb at a level of 0.85 ppm,
which is above the action limit. Beta Labs would submit “Subcontracted test” as the test type whereas
Alpha Labs would indicate that pesticides failed and identify Aldicarb as the specific analyte with the
detected level in parts per million.
Figure 20: Primary Lab Entering Pass/Fail Results on Subcontracted Test
29
Testing Inhalable Hemp Vapor Items from non-OLCC licensees
Senate Bill 96, passed in the 2021 Oregon Legislative Session, requires the Commission to regulate
testing and labeling of inhalant delivery systems that contain cannabinoids derived from industrial
hemp. Persons who are creating and selling hemp vapor items outside of the recreational
marijuana system will now be required to conduct tracked compliance testing on those items.
When a lab is receiving an inhalable hemp vapor item from a source outside of Metrc they will
generally follow the process as outlined within the
https://wiki-or.metrc.com/transfers/seed-
transfers with some differences as outlined in this section. A laboratory will log into metrc and
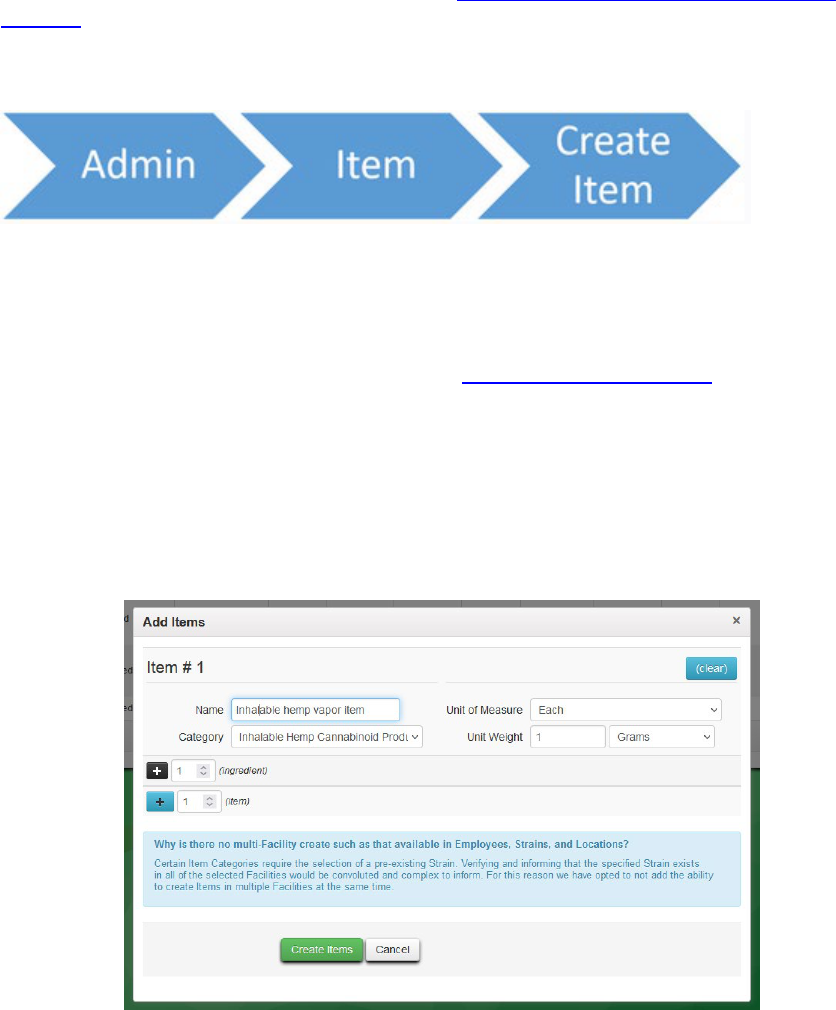
create a new item:
Figure 21: metrc path to create an item
The laboratory should create the item they will be intending to receive from the non-Metrc
source. In the category section the laboratory should ensure it reflects the correct item type that
will be tested (either “Hemp Extract,” “Hemp Concentrate,” or “Inhalable Hemp Cannabinoid
Product (with non-cannabis additives)”). For questions about the differences between these
categories, and when to use which one, see OLCC’s Product Classification Guide
.
It is satisfactory for a laboratory to create a single “catch-all” item, so long as the categorization of
the item is correct. For example, a laboratory could create three generic hemp vapor items (one as
“Hemp Extract,” one as “Hemp Concentrate,” and one as “Inhalable Hemp Cannabinoid Product
(with non-cannabis additives)”), without needing to specify e.g. “Acme Products Hemp Vapor
Item” versus “Omega Products Hemp Vapor Item.”
Figure 22: Add items window
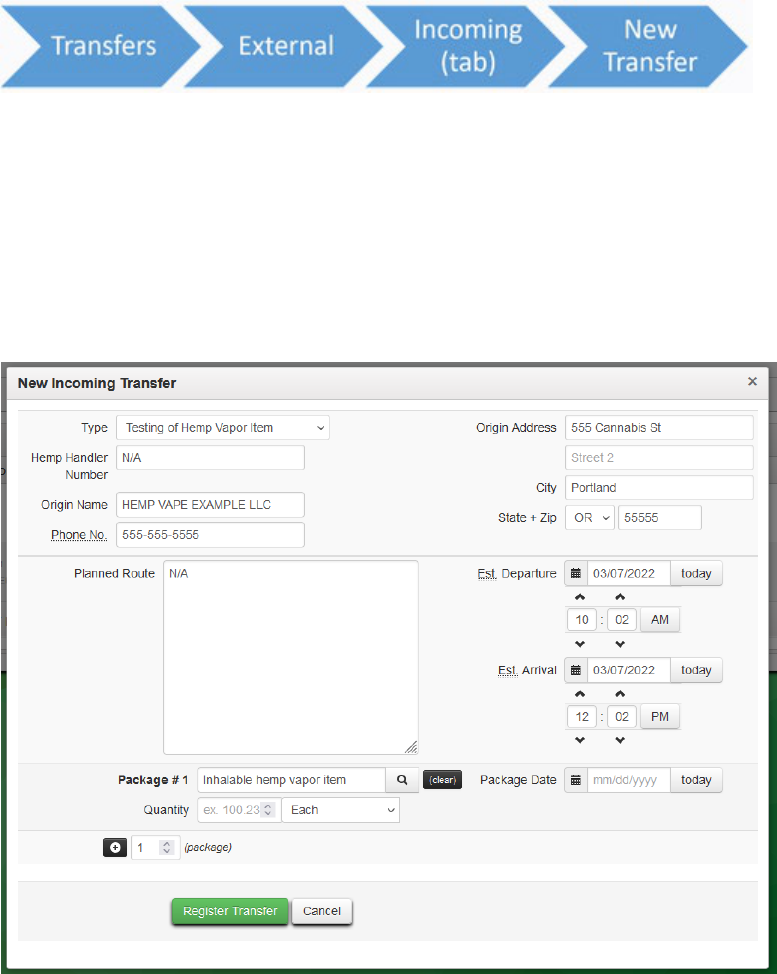
The laboratory should then create an external manifest and select the “Testing of Hemp Vapor
30
Item” type:
Figure 23: Metrc path to create an external transfer
The laboratory should they fill out the manifest with the appropriate information.
The Hemp Handler information can be entered as “N/A” if the source is not from an ODA Hemp
Handler. However, if the person or business is an ODA registered Hemp Handler, their handler
registration number would be entered. In the package section the laboratory should select the
item that they created in the first part of this section.
Figure 24: Edit the manifest information
The final steps to bringing in products on an external transfer are to accept the transfer and assign
the packages a UID number and a location in Metrc. Once this process is complete, the newly
created packages will move to your active packages area in Metrc. From the Incoming External
Transfers Tab, select the manifest that was created in the steps above. Then click the "complete"
button:
Figure 25: Complete the Manifest
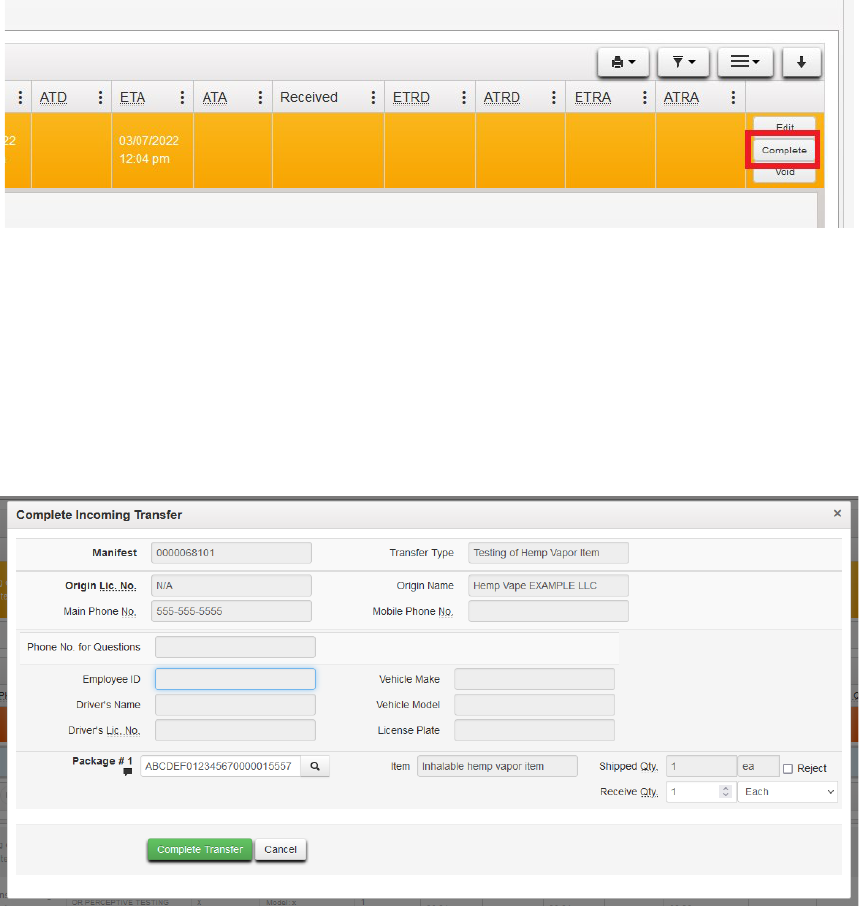
31
The following dialog box opens. Where indicated, select a package UID and the package location
at your facility. You may click on the magnifying lens for a list of tags/locations or you may begin
typing to have Metrc provide autofill options. Continue this process for all packages on the
manifest. Then click Complete Transfer to move the package(s) to your active inventory, at which
point the package is in your inventory and may have test results entered, as explained elsewhere
in this guide.
Figure 25: Complete incoming transfer

32
Appendix 1: Frequently Asked Questions
I want to combine multiple packages into one batch for purposes of testing – can I submit them for
testing together?
If the multiple packages would meet all the other requirements of what constitutes a “batch”
when combined, then yes, you can submit multiple packages together under one sample as a
single batch for purposes of testing. Read OHA’s testing rules
to ensure you understand and
meet these requirements. To draw one sample from multiple packages in Metrc, add each
Individual p
ackage as “contents” on the right-hand side of the Submit for Testing box when you
are creating a sample. Keep in mind that if a single sample drawn from multiple packages fails
for any test all packages from which the sample is drawn will also fail. In other words,
combining packages into a single sample may result in fewer tests being run but there may be
greater risk that the combined product will fail.
Is a lab able to conduct “extra” tests like terpenes not covered by the testing rules? How should the
sampling and test results be recorded in Metrc?
If a test is requested that is not part of the state-required testing regime, it can be conducted by
a lab even if that lab is not ORELAP-accredited for the specific test. For example, if a producer
requests a test for terpenes, any OLCC-licensed lab may conduct that test. However, a
laboratory cannot identify the test results as accredited results.
However, these “extra” tests need to be handled differently in Metrc. First, the test result
should not be entered into Metrc. Results of these tests should be communicated to clients
through some other form.
Second, even though the test results will not be entered into Metrc, the chain of custody must
still be documented in Metrc. The transfer of product from the requesting licensee to the lab
must be conducted in Metrc via a transfer manifest.
Third, do not submit a batch for testing for these “extra” tests. If these optional tests are the
only ones being sampled for, create a standard (non-sample) package with the appropriate
weight and transfer it to a lab. Once testing is completed, the lab will adjust the package with
the appropriate reason code, but no test results will be entered. If you create a sample package
for a batch undergoing “extra” testing it will block required test results from transmitting to the
originating batch (see the question below). Alternatively, if you submit failing results for these
“extra” tests, it will fail the batch as a whole and block the producer or processor from selling
the batch through the supply chain.
Can a lab test for potency as part of quality control and not have it “count” for purposes of state
testing? What about for pesticides?
A processor may opt for Research and Development testing for any test, and a producer may
elect to have R&D testing for anything other than pesticides. See the Research and Development
Testing section of this guide for more information.

33
Test results were entered, but the status still says “TestingInProgress” – why?
The final testing status will not be determined until all sample packages taken from the same
source have had test results submitted. For example, if two sample packages are created from
one harvest, after the first sample is tested the status of the harvest and both sample packages
will become “TestingInProgress.” After test results for the second sample are submitted the
status of the harvest and sample packages will update to reflect the test results (either
“TestPassed” if all tests on both samples passed, or “TestFailed” if any test on either sample
failed). If you believe that all samples have been tested and the status of “TestingInProgress” is
incorrect, please contact Metrc customer support.
A lab tested my sample and gave me a PDF report of my test results, but my package in Metrc still says
“NotSubmitted” – can I still transfer my product?
No. For lab testing to meet state requirements for OLCC licensees test results must be entered
into Metrc and the batch’s Metrc testing status must indicate that it has passed testing. If the
sampling and testing occurred outside of Metrc it must be resampled and retested properly. If
the sampling and testing did occur within Metrc but the batch’s testing status was not updated,
it is possible that the sample was recorded incorrectly. If you believe this to be the case, contact
Metrc support at support@metrc.com
or (877) 566-6506
My product failed a test. What are my options?
If the test that failed was water activity, solvents, potency, or microbiological contamination,
you have two options: retesting or remediation. To retest, you must pass two additional times,
once by the original lab and a second confirmation test by a new lab. To remediate you must
choose a method allowed for under rule; you will have to submit the product for testing again
once it’s been remediated. You may remediate after the first failed test or if the retesting
process has resulted in a second failure.
If you are a producer and your product failed for pesticides, your only option is retesting –
remediation for pesticides is not allowed. If it fails the retesting process, the batch must be
destroyed.
See the Retesting and Remediation section of this guide for more details. See Appendix 3 for
further details regarding remediation.

34
My harvest lot has more than one batch, and each batch has a slightly different THC content – how
should I label my product?
The OLCC labeling rules require the label to list the average THC and CBD of all tests conducted
for that physical material being sold. In this example, if each of these batches were packaged
and sold separately, their batch-specific potency results would need to be on the label. If,
however, the batches from the same harvest lot were co-mingled for sale, the label would list
the average of the potency results for the batches that were combined.
If a lab goes out to sample at the site of a producer or processor how should the data be recorded in
Metrc?
OHA’s testing rules and ORELAP accreditation require that only OLCC-licensed labs accredited
for sampling are able to take samples for state-required tests. If a lab is sampling at the site of a
producer or processor, the product will still be in this licensee’s Metrc inventory when the lab
arrives to take samples. The lab should take physical samples according to OHA and ORELAP
requirements and then instruct the producer or processor to create sample packages in the
producer or processor’s Metrc account with the appropriate weight(s). The sample(s) should
then be transferred via transfer manifest to the lab or labs conducting the testing. When the
sample arrives on-site at the lab, the sample should be weighed to verify the information
entered into Metrc and the manifest should be accepted into the lab’s inventory.
If a producer brings in a 50.0 pound batch for sampling how should this be recorded in Metrc?
Under OLCC rules, labs may accept whole batches from producers and sample directly at the lab
location. In Metrc, the producer would transfer the entire batch to the lab without submitting
for testing. The lab would receive the transferred batch into their inventory. Once in the lab’s
inventory, the lab would submit the batch for testing and create the sample package with the
appropriate weight. This requires the lab to have their own package tags in stock. Once the
sample package is created, the lab can choose to either keep the remainder of the batch in their
own inventory, transfer it back to the originating producer, or transfer it to an OLCC-licensed
wholesaler while the test results are pending. It should be noted that 50.0 pound batches may
be submitted on and after July 1, 2022.
Can samples be stored by a lab at a remote, unlicensed facility?
All marijuana items must be stored on a licensed premises. A remote office, if unlicensed, could
not be used to store marijuana items. If a remote office is being used as a waypoint as part of
transportation of samples or other marijuana items, OLCC must be notified in advance if the
stop is longer than two hours. While stopped at an unlicensed location all marijuana items,
including samples, must remain secured in the transporting vehicle until the driver reaches the
final, licensed destination.
Samples and other marijuana items can be stored at any licensed premises, not only the lab’s
own premises, as long as it is properly documented within Metrc according to OLCC rules.
Therefore, if, for example, a sampling lab is too far away from its own licensed premises to store
materials overnight, the samples could be instead stored with an OLCC-licensed wholesaler.

35
If I am a licensed lab can I have my employees collect samples?
If a lab is accredited by ORELAP for sampling and licensed with the OLCC, any employee at the
lab may collect samples. All physical sampling must be done in accordance with OHA rules
and
ORELAP standards and must be accurately reflected in Metrc.
Are there options other than manual data entry for inputting information into Metrc?
For both package adjustments and test results there are two alternatives to manual data entry:
an Application Program Interface (API) and CSV upload.
Metrc has developed an API that can be used by third-party software, such as LIMS, to enter
information into Metrc automatically. Companies must first be credentialed for use within
Metrc and be issued an API key. A list of the software companies that have passed this
certification process can be found on the Metrc website. Although most functions within Metrc
will be supported in the API, creating transfers between facilities will need to be done manually
within Metrc.
For labs using third-party software that has not developed an API, CSV upload is another
alternative for data entry. The required information for a data upload differs based on the data
being imported (e.g. adjustment vs. lab test results), and you should read the relevant
documentation to ensure that all information is included. One common requirement is the
package RFID number – if you are able to track this number in your LIMS and your LIMS supports
data export to Excel or CSV the data upload to Metrc should be a feasible option to avoid
manual data entry.
How many scales do I need? Do they need to be certified?
The number and types of scales you will need depends on what types of weighing you will be
doing. OLCC rules require that any weights entered into Metrc must be derived from a scale
certified by the appropriate state agency. For labs, ORELAP certification is sufficient for any
scales covered by the ORELAP accreditation process, such as field sampling scales. For all other
license types (e.g. producers, processors, retailers, etc.) and for lab scales not directly used for
sampling, scales must be certified by ODA.
By and large, if a lab will not accept entire batches into their inventory and will only sample at
the premises of a producer, processor, or wholesaler, the lab will not need any scales other than
those certified by ORELAP. However, for labs accepting entire batches from a producer into their
inventory, those sampling scales certified by ORELAP will not have sufficient capacity and the lab
will need one or more ODA certified scales.
Where can I go or who should I ask if I have more questions?
For questions regarding rules and requirements you should check marijuana.oregon.gov.
You
can also subscribe to email alerts from OLCC. If you have questions that are not answered by
reading the materials posted online, you can contact the OLCC at marijuana@oregon.gov or
[email protected] or (503) 872-5000.

36
For questions regarding how to use Metrc, the Metrc support desk can provide assistance for
questions related to specific functionality or troubleshooting. They can be contacted at
support@metrc.com
or (877) 566-6506.
For questions related to how specific scenarios not covered in this guide should be recorded in
Metrc, contact OLCC’s Marijuana Technical Unit at marijuana.[email protected]
or (503) 872-
5190.
37
Appendix 2: Testing Requirements
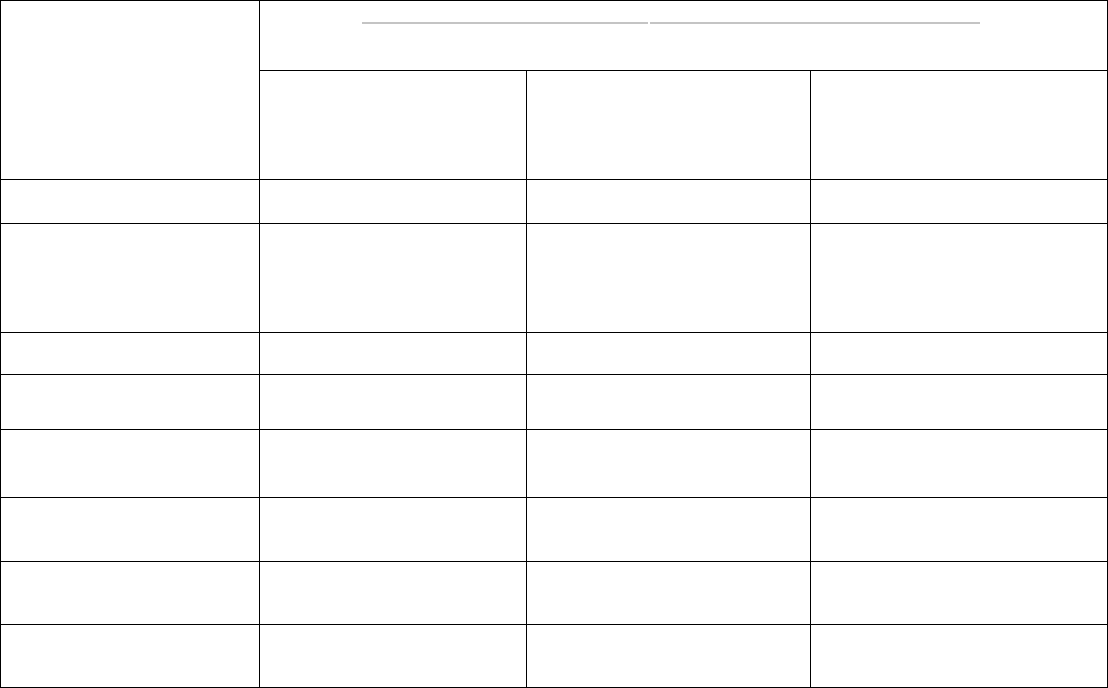
OAR 333-007-0320 Compliance Testing Requirements for Marijuana or Usable Marijuana
Testing Requirements
Marijuana or Usable Marijuana
If intended for sale from a
retail shop or dispensary
If intended for further
processing into a product
If intended for further
processing into a concentrate,
extract, or finished inhalable
cannabinoid product
Pesticides Yes Yes No
Water Activity / Moisture
Content
Yes Yes
Yes, if harvested before March
1, 2023 unless processor will
use a process that results in
effective sterilization
Solvents No No No
Delta-9 THC, Delta-9
THCa and CBD
Yes No No
Delta-8 THC
Yes, on and after July 1,
2022
No No
Mycotoxins
Yes, if harvest on and after
July 1, 2022
Yes, if harvest on and after
July 1, 2022
No
Heavy Metals
Yes, if harvest on and after
March 1, 2023
Yes, if harvest on and after
March 1, 2023
No
Microbiological
Contaminants
Yes, if harvest on and after
March 1, 2023
Yes, if harvest on and after
March 1, 2023
No
NOTES:
• A marijuana producer must test every batch from a harvest lot of marijuana or usable marijuana harvested before
March 1, 2023 intended to produce kief for water activity prior to producing the kief unless the producer tests the
kief for water activity per OAR 333-007-0420.
• On and after July 1, 2022, a producer or grower may separate each harvest lot of marijuana or usable harvested
into no larger than 50.0-pound batch. In addition, the definition of a harvest lot is being expanded from 72 hours to
seven calendar days.
38
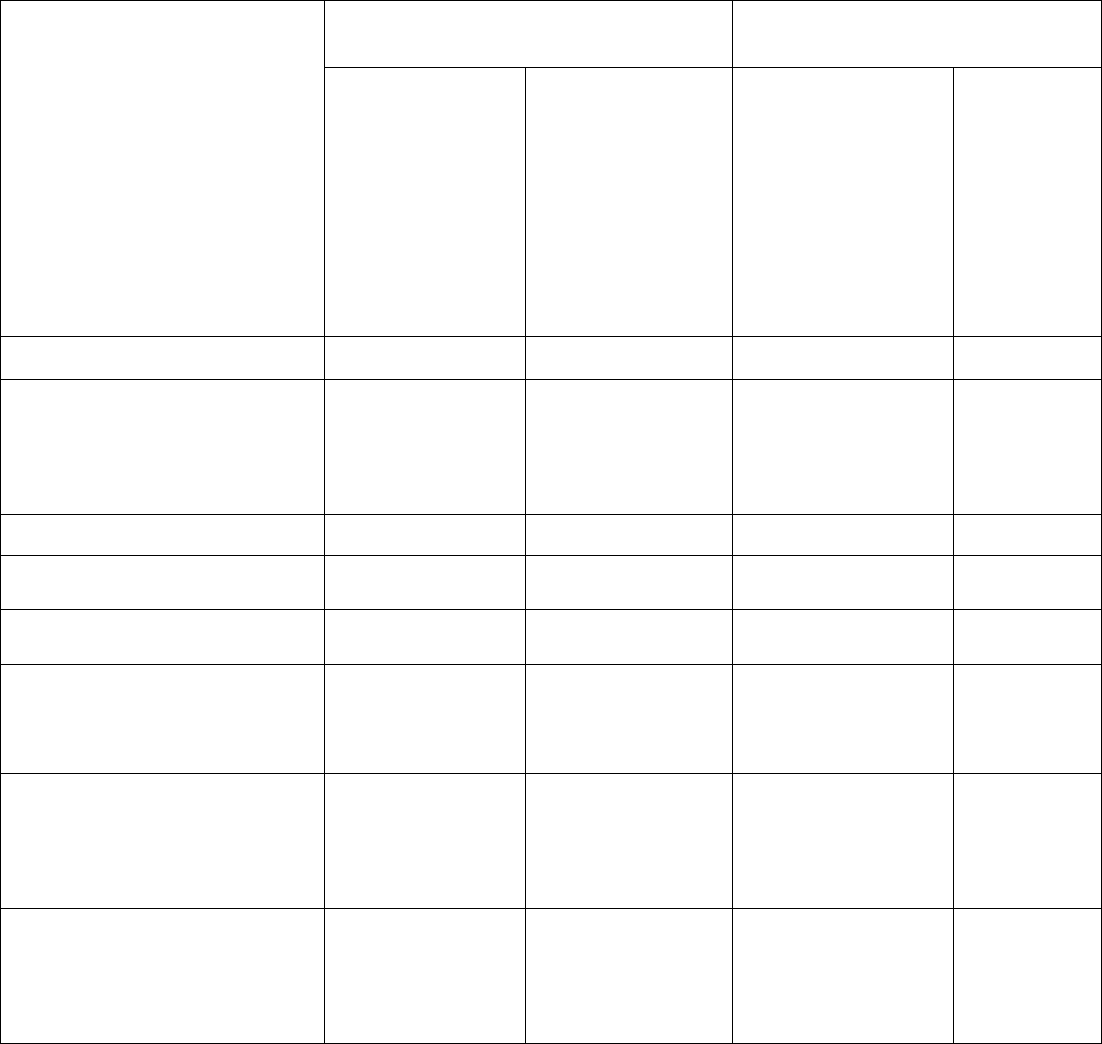
OAR 333-007-0330 Compliance Testing Requirements for Cannabinoid Concentrates and Extracts
Testing Requirements
Concentrate or Extract
Producer producing kief as permitted
under OAR 845-025-2020
If intended for sale
from a retail shop
or dispensary
If intended for
further processing
into a product,
except a finished
inhalable
cannabinoid
product
If intended for sale
from a retail shop or
dispensary
If intended
for further
processing
into a
product,
except a
finished
inhalable
cannabinoid
product
Pesticides Yes Yes Yes Yes
Water Activity / Moisture
Content
No No M
Yes, if
manufactured
on or before
March 1,
2023
Solvents Yes* Yes* No No
Delta-9 THC, Delta-9 THCa and
CBD
Yes No Yes No
Delta-8 THC
Yes, on and after
July 1, 2022
No
Yes, on and after July
1, 2022
No
Mycotoxins
Yes, if
manufactured on
and after July 1,
2022
Yes, if manufactured
on and after July 1,
2022
Yes, if manufactured
on and after July 1,
2022
Yes, if
manufactured
on and after
July 1, 2022
Heavy Metals
Yes, if
manufactured on
and after March 1,
2023
Yes, if manufactured
on and after March
1, 2023
Yes, if manufactured
on and after March 1,
2023
Yes, if
manufactured
on and after
March 1,
2023
Microbiological Contaminants
Yes, if
manufactured on
and after March 1,
2023
Yes, if manufactured
on and after March
1, 2023
Yes, if manufactured
on and after March 1,
2023
Yes, if
manufactured
on and after
March 1,
2023
*A processor is exempt from testing for solvents if they did not use any solvent listed in OAR 333-007-0410, Table 4 and
either only used a mechanical extraction or used only water, animal fat, or vegetable oil as a solvent.
NOTE: A cannabinoid concentrate made only using food grade animal fat or food grade plant-based oil is not required to be
tested for pesticides, mycotoxins starting July 1, 2022, heavy metals starting March 1, 2023 or microbiological contaminant
starting March 1, 2023 if the marijuana used to make the concentrate was tested and passed for pesticides, mycotoxins on
or after July 1, 2022, heavy metals on or after March 1, 2023 or microbiological contaminant on or after March 1, 2023.

39
OAR 333-007-0340 Compliance Testing Requirements for Cannabinoid Products
Testing Requirement Finished cannabinoid product prior to selling or transferring
Pesticides No
Water Activity / Moisture Content No
Solvents No
Delta-9 THC, Delta-9 THCa and CBD Yes
Delta-8 THC Yes, on and after July 1, 2022
Mycotoxins No
Heavy Metals No
Microbiological Contaminants No
NOTE: Any marijuana, usable marijuana, concentrate or extract must be fully tested and pass prior to being used to make a
cannabinoid product. OAR 333-007-0341 Compliance Testing Requirements for Finished Inhalable Cannabinoid Products
Testing Requirement Finished inhalable cannabinoid product prior to selling or transferring
Pesticides Yes
Water Activity / Moisture Content No
Solvents Yes*
Delta-9 THC, Delta-9 THCa and CBD Yes
Delta-8 THC Yes, on and after July 1, 2022
Mycotoxins Yes, if manufactured on and after July 1, 2022
Heavy Metals Yes, if manufactured on and after March 1, 2023
Microbiological Contaminants Yes, if manufactured on and after March 1, 2023
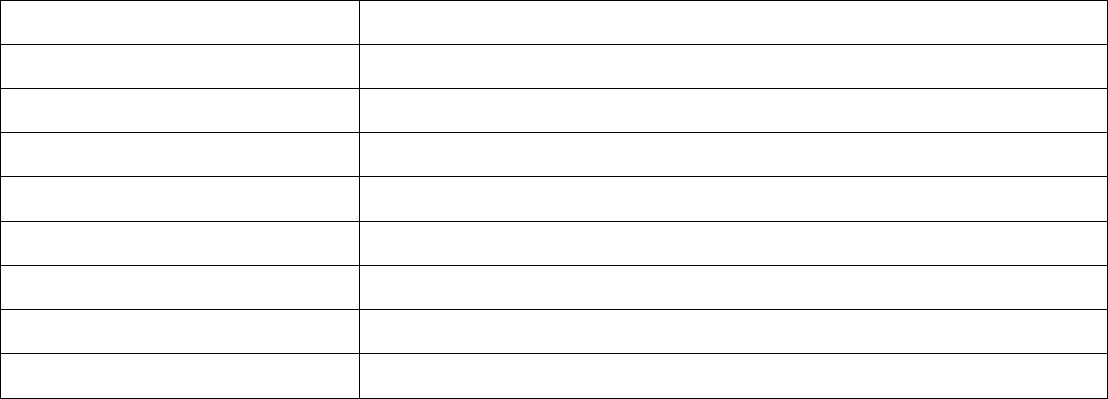
40
OAR 333-007-0342 Compliance Testing Requirements for Industrial Hemp-Derived Vapor Item
Testing Requirement Industrial hemp-derived vapor item product prior to selling or transferring
Pesticides Yes
Water Activity / Moisture Content No
Solvents Yes*
Delta-9 THC, Delta-9 THCa and CBD Yes
Delta-8 THC Yes, on and after July 1, 2022
Mycotoxins Yes, if manufactured on and after July 1, 2022
Heavy Metals Yes, if manufactured on and after March 1, 2023
Microbiological Contaminants Yes, if manufactured on and after March 1, 2023
*A processor is exempt from testing for solvents if they did not use any solvent listed in OAR 333-007-0410, Table 4 and
either only used a mechanical extraction or used only water, animal fat, or vegetable oil as a solvent.
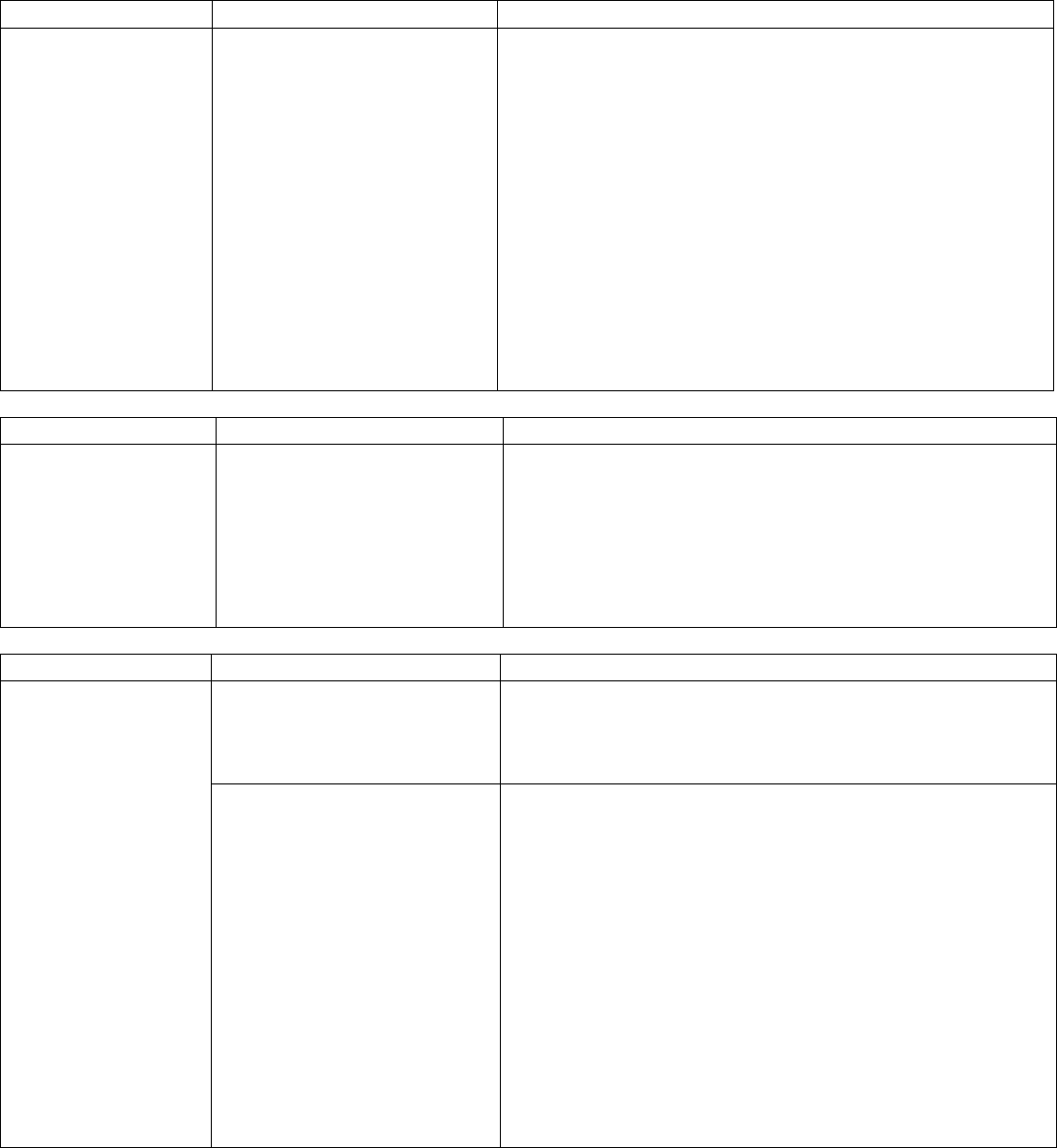
41
Appendix 3: Cannabis Failed Testing Guide
Rules around failed tests may be found under OAR 333-007-0450.
Test Failed
Marijuana Item Type
Action
Water activity or
moisture content
Marijuana or Usable marijuana
Batch may either:
• Be used to make a concentrate or extract if the
processing method effectively sterilizes the batch; OR
• Undergo additional drying or curing and be resampled
and tested for pesticides, water activity and moisture
content, adult use cannabinoids and CBD, heavy
metals if the marijuana or usable marijuana was
harvested on or after March 1, 2023, mycotoxins if the
marijuana or usable marijuana was harvested on or
after July 1, 2022, and microbiological contaminates if
the marijuana or usable marijuana was harvested on
and after March 1, 2023.
Test Failed
Marijuana Item Type
Action
Solvents Extract
• Batch may be remediated.
• A remediated batch must be retested for pesticides,
solvents and potency. If manufactured on or after July 1,
2022 mycotoxins. If harvested on or after March 1, 2023
heavy metals and microbiological contaminates.
• A batch that fails solvent testing that is not remediated, or
if remediated fails testing, must be destroyed.*
Test Failed
Marijuana Item Type
Action
Heavy metals
Marijuana, usable marijuana,
finished inhalable cannabinoid
product, or industrial hemp-
derived vapor item
May not be remediated and must be destroyed
Concentrate or extract
• May be remediated using procedures that would
reduce the concentration of heavy metals to less than
the action level
• Must be re-sampled and re-tested for pesticides,
solvents if applicable, potency, heavy metals,
mycotoxins if manufactured on and after July 1, 2022,
and microbiological contaminates if manufactured on
and after March 1, 2023.
• A batch that fails heavy metal testing that is not
remediated or that fails testing after remediation
must be destroyed in a manner specified by the
Authority, Commission, or the Department of
Agriculture.
42
Test Failed
Marijuana Item Type
Action
Mycotoxins
Marijuana item or industrial
hemp-derived vapor item
May not be remediated and must be destroyed
Test Failed
Marijuana Item Type
Action
Microbiological
Contaminants
Marijuana or usable marijuana
• Be remediated using a sterilization process; OR
• Be used to make a concentrate or extract, if processing
method effectively sterilizes the batch; OR
• The batch may be destroyed. *
After remediation or further processing must be tested for
microbiological contaminants, solvents (if required) and
pesticides, mycotoxins if harvested or manufactured on or
after July 1, 2022, heavy metals if harvested or
manufactured on or after March1, 2023 heavy metals. If
batch fails retesting, the batch must be destroyed. *
Concentrate, extract,
finished inhalable cannabinoid
product or industrial hemp-
derived vapor item
• Batch may be further processed.
• After further processing, batch must be retested for
microbiological contaminants, solvents (if required) and
pesticides, mycotoxins if harvested or manufactured on
or after July 1, 2022, heavy metals if harvested or
manufactured on or after March1, 2023 heavy metals. If
batch fails retesting, the batch must be destroyed. *
All other products Must be destroyed. *
Test Failed
Marijuana Item Type
Action
Potency
Extracts, Concentrates, and
Cannabinoid products
Product may be repackaged to meet concentration levels
outlined in rule. A repackaged item must be resampled and
retested. A marijuana item or industrial hemp-derived vapor
item that is re-mixed due to failing potency must be re-
sampled and retested for pesticides, potency, solvents if
applicable, mycotoxins if manufactured on or after July 1,
2022, heavy metals if manufactured on or after March 1,
2023 and microbiological contaminates in manufactured on
or after March 1, 2023.
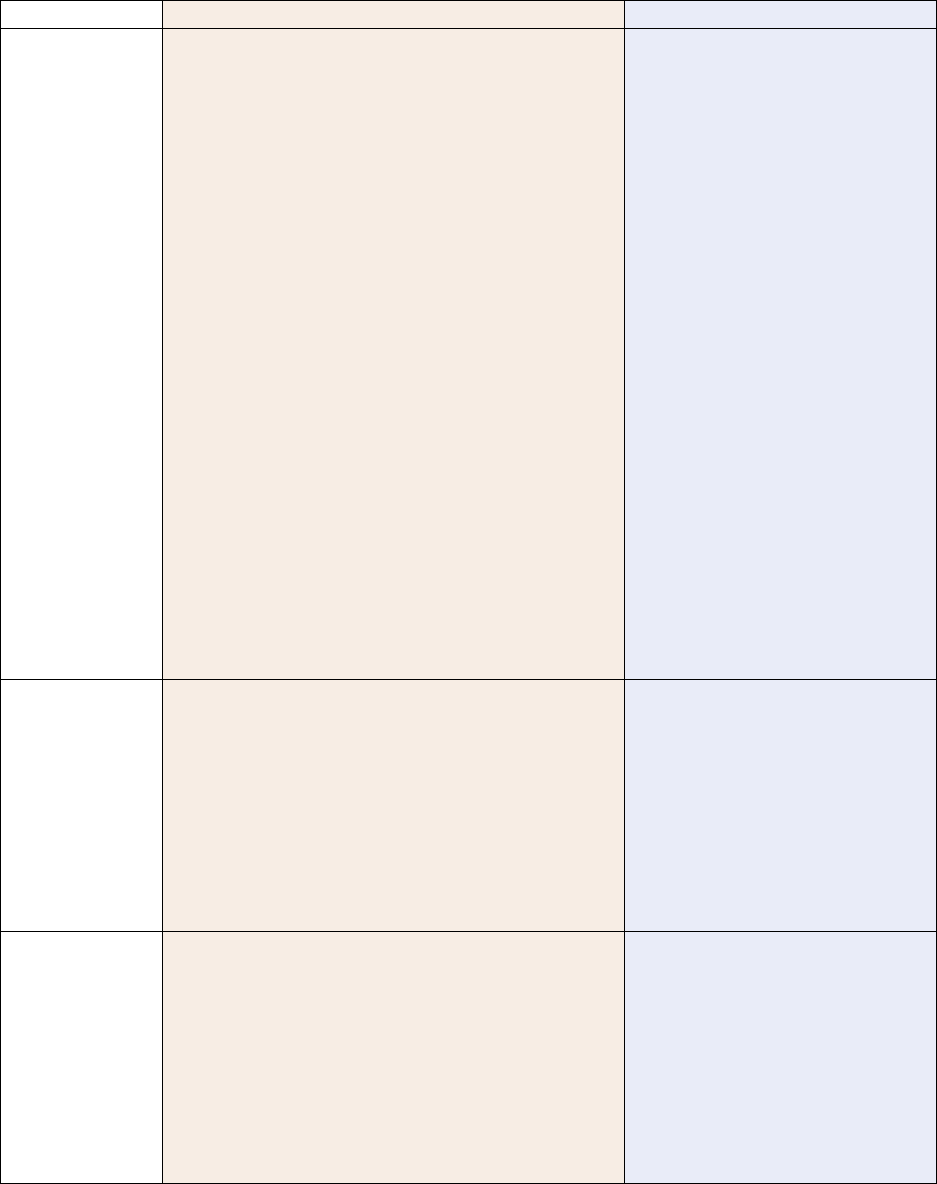
43
Appendix 4: How to Enter Test Results
Test Category
How to enter result
If result is failure…
Potency
First, select “Potency (pass/fail)” and select pass
or fail as the Status (no specific value is
required). You should select fail as the Status if
potency fails for any of the reasons as outlined in
rule. Regardless of whether Potency passes or
fails, you should also enter the Total THC and
Total CBD amounts.
Second, select “Total THC (mg/g),” select pass or
fail as the Status, and enter the milligrams of
Total THC per gram.
Third, select “Total CBD (mg/g),” select pass as
the Status, and enter the milligrams of Total CBD
per gram.
Fourth, Select “Delta 8 THC (mg/g)” select pass
as the Status, and enter the milligrams of Delta 8
THC per gram.
Fifth, for non-usable marijuana only, select
“THC (%RSD)” or “THC (RPD)” as relevant and
enter the value calculated. This should be
done for CBD and Delta 8 THC as well.
No additional information
required; Total CBD cannot fail
but is a required test for
informational purposes
[Potency and Total THC can fail].
Pesticides
Select “Pesticides (pass/fail)” and select pass or
fail as the Status.
If specific analyte is detected above LOQ: select
the specific analyte that was detected and enter
the specific value detected as parts per million in
the Quantity field.
Select the specific analyte(s)
that failed, select “Failed” as the
Status, and enter the specific
value detected as parts per
million in the Quantity field.
Heavy Metals
Select “Heavy Metals” (pass/fail)” and select
pass or fail as the Status.
If specific analyte is detected above LOQ: select
the specific analyte that was detected and enter
the specific value detected as parts per million
in the Quantity field.
Select the specific analyte(s)
that failed, select “Failed” as the
Status, and enter the specific
value detected as parts per
million in the Quantity field.
44
Mycotoxin
Select “Mycotoxin” (pass/fail)” and select pass
or fail as the Status.
If specific analyte is detected above LOQ: select
the specific analyte that was detected and enter
the specific value detected as parts per million
in the Quantity field.
Select the specific analyte(s)
that failed, select “Failed” as the
Status, and enter the specific
value detected as parts per million
in the Quantity field.
Solvents
First, select “Solvents (pass/fail)” and select pass
or fail as the Status. If the result is a pass, you do
not need to enter a specific test quantity.
Second, select “Solvents (RPD)” and enter the
value calculated.
Select the specific analyte(s)
that failed, select “Failed” as the
Status, and enter the specific
value detected as microgram
per gram in the Quantity field.
Water Activity
Select “Water Activity (Aw),” select pass or fail as
the Status, and enter the partial vapor pressure
value detected.
No additional information
required.
Micro
biological
Contaminants
Select “Mycotoxin” (pass/fail)” and select pass or
fail as the Status.
If a specific analyte is detected select the analyte
and select pass or fail as the Status.
Select the specific analyte(s)
that failed, select “Failed” as
the
Status
45
Moisture
Content
Select “Moisture Content (%),” select pass or fail
as the Status, and enter the moisture content of
the sample detected as a percentage.
No additional information
required.
Microbiological
Contamination
Select “Coliforms (#),” select pass or fail as the
Status, and enter the total coliform count
detected.
If coliforms are detected, select
“E. Coli (CFU/g),” select pass or
fail as the Status, and enter the
coliform-forming unit per gram
detected.
Subcontracted
Tests
Select “Subcontracted test” type that matches
the test type the subcontracted lab is
conducting. The subcontracted lab will enter
this information and the primary lab will enter
the results for the specific test conducted (e.g.
pesticides) and will upload the complete
Certificate of Analysis. For this test “pass”
simply means that the test was conducted and
does not have any bearing on whether the test
conducted was above or below the action limit.
No additional information
required.
Tentatively
Identified
Compounds
Select “Tentatively Identified Compounds
(cannot fail),” select “Passed” as the status, and
in the notes field indicate the (up to) five
Tentatively Identified Compounds that were
detected
Tentatively Identified
Compounds cannot fail, but is a
required test for informational
purposes.
